Hijacking the cell machinery allows rapid studies of peptide variants to expose mechanisms of action
Credit: UCI School of Medicine
Irvine, Calif. – March 4, 2020 – In the past, biologically-active peptides – small proteins like neurotoxins and hormones that act on cell receptors to alter physiology – were purified from native sources like venoms and then panels of variants were produced in bacteria, or synthesized, to study the structural basis for receptor interaction. A new technique called zombie scanning renders these older processes obsolete.
Peptides are used for medical therapy and to study biology, among other things, but their production cost in time and money is increasingly high.
“If a peptide has 30 residues, simply changing each site once requires the synthesis, purification and validation of the folded composition of all 30 variants, a process that requires months and many thousands of dollars,” said Steven A.N. Goldstein, MD, PhD, vice chancellor for Health Affairs at the University of California, Irvine, and distinguished professor in the UCI School of Medicine Departments of Pediatrics and Physiology & Biophysics.
Published today in Science Advances, the new study co-led by Goldstein and Jordan H. Chill, PhD, a professor in the Department of Chemistry at Bar-Ilan University in Israel, reveals how researchers were able to hijack cell machinery to simplify the creation of peptides allowing for extensive, rapid studies of structure-function and mechanism to improve specificity and affinity of action, the important parameters for therapeutic efficacy.
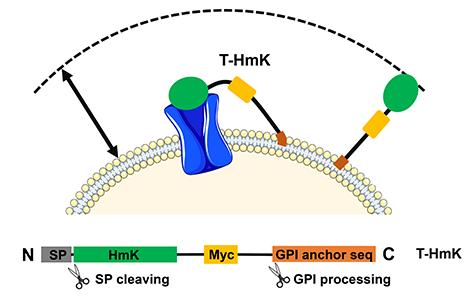
“Since we hijack the cell machinery to synthesize and display the peptides on the cell surface with the receptor, we dub this zombie scanning,” said first author Ruiming Zhao, PhD., an investigator in the Goldstein laboratory.
“Using this new technique, peptide changes are as simple as plasmid synthesis and require only days of work and pennies per construct. This enables us to study the roles of many sites with many changes in a much shorter period of time at a much lower cost.”
The study, titled, “Tethered peptide neurotoxins display two blocking mechanisms in the K+ channel pore as do their untethered analogues,” outlines how the encoded peptides are linked via a native pathway to the outside of the cell on a flexible tether. In this case, the receptor target was also expressed from a plasmid and could be modified. The method also allows study of low affinity interactions that would not otherwise be feasible to analyze.
Using zombie scanning, researchers made the unexpected discovery that a peptide in clinical trials as an immune suppressant acts differently than once thought, revealing that this family of neurotoxins has two possible modes of interacting with potassium channels rather than just one.
Chill and colleague Netanel Mendelman, PhD, enhanced the impact of these findings by elucidating the three-dimensional structure of a selected neurotoxin using nuclear magnetic resonance (NMR).
“By estimating hundreds of distances and angles between atoms in the peptide, we now know its structure, offering a molecular context for these exciting results,” said Chill. “The two binding modes seem to involve a ‘flipping’ of the toxin or some rearrangement of its atomic structure.”
Alternative binding modes as described for these peptides are a troublesome confounding factor in structure-based drug design, highlighting the importance of the findings of this report and future studies of the recognition process between channels and inhibitory peptides.
###
The work was funded in part by a grant for the National Institutes of Health and the US-Israel Binational Science Foundation.
About Bar Ilan University Department of Chemistry
Bar Ilan University, Israel’s second-largest academic institution, is located in the Tel-Aviv metropolitan area. The Department of Chemistry is home to 35 active research groups and over 150 graduate students and post-doctoral fellows working in all fields of chemistry. A third of these are affiliated with the Bar Ilan Institute for Nanotechnology and Advanced Materials (BINA), fostering advanced scientific achievements in the areas of nano-materials, nano-medicine, nano-photonics and energy. The Department maintains strong efforts in material science and in the chemical biology field, the latter including structural biology, peptide chemistry, bio-organic chemistry, drug design and computational chemistry.
About UCI Health Affairs
UCI Health Affairs comprises the schools, institutes, and centers in the Susan and Henry Samueli College of Health Sciences and an academic health system, UCI Health. The college unites the disciplines of medicine, nursing, pharmacy and pharmaceutical sciences, and population and public health to advance a transformative educational and healthcare delivery model that is patient-centered, science-based, transdisciplinary, and team-delivered.
About the UCI School of Medicine
Each year, the UCI School of Medicine educates more than 400 medical students, and nearly 150 doctoral and master’s students. More than 700 residents and fellows are trained at UCI Medical Center and affiliated institutions. The School of Medicine offers an MD; a dual MD/PhD medical scientist training program; and PhDs and master’s degrees in anatomy and neurobiology, biomedical sciences, genetic counseling, epidemiology, environmental health sciences, pathology, pharmacology, physiology and biophysics, and translational sciences. Medical students also may pursue an MD/MBA, an MD/master’s in public health, or an MD/master’s degree through one of three mission-based programs: the Health Education to Advance Leaders in Integrative Medicine (HEAL-IM), the Leadership Education to Advance Diversity-African, Black and Caribbean (LEAD-ABC), and the Program in Medical Education for the Latino Community (PRIME-LC). The UCI School of Medicine is accredited by the Liaison Committee on Medical Accreditation and ranks among the top 50 nationwide for research. For more information, visit som.uci.edu.
Media Contact
Anne Warde
[email protected]
949-824-6357
Original Source
https:/
Related Journal Article
http://dx.




