Funding will support combined use of recent Nobel Prize-winning technology with a powerful new imaging method; lays groundwork for detecting, treating underlying causes of Alzheimer’s, Parkinson’s and Lewy body dementia
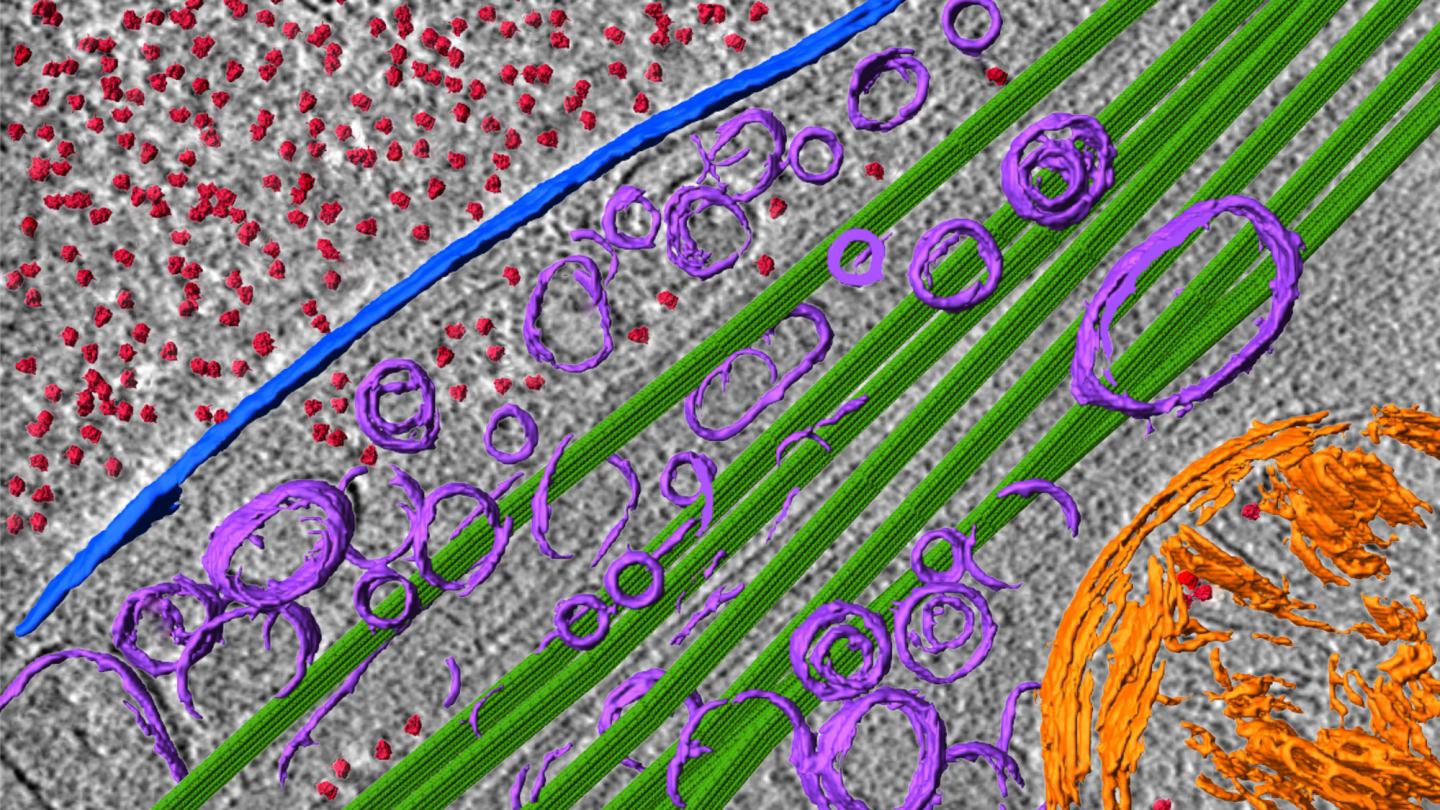
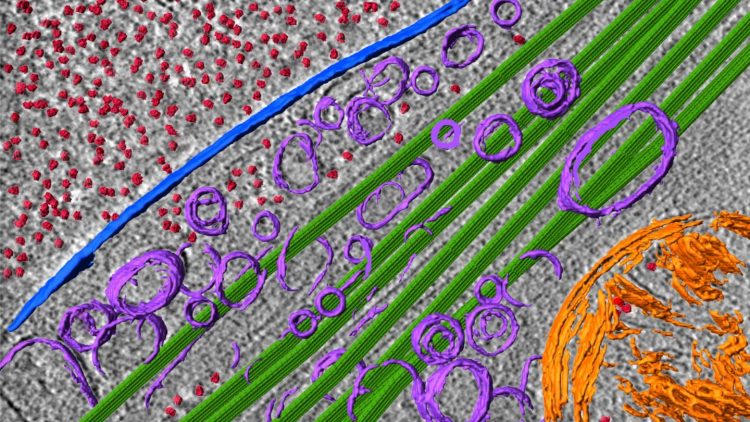
Credit: Fitzpatrick lab/Columbia’s Zuckerman Institute
NEW YORK — Anthony Fitzpatrick, PhD, a Columbia scientist and Zuckerman Institute principal investigator, is part of a team of researchers selected by the NIH to map the three-dimensional structure of proteins linked to Alzheimer’s disease, Parkinson’s disease and Lewy body dementia, a trio of progressive and fatal neurodegenerative disorders for which there are few treatments and no cure.
This award of $2.84 million will enable Dr. Fitzpatrick to bring together for the first time two revolutionary imaging technologies meant to reveal the structures and behaviors of toxic proteins believed to drive these disorders. Using single-particle cryo-electron microscopy (cryo-EM), a technology that shared the Nobel Prize in Chemistry in 2017, Dr. Fitzpatrick and his team plan to image the proteins in 3D at an atomic scale. An increasingly powerful technology called cryo-electron tomography (cryo-ET) will then help the scientists to understand the cellular environments in which these proteins function.
The grant, administered by the NIH’s National Institute of Neurological Disorders and Stroke, encompasses several projects and is led by co-principal investigators Dennis Dickson, MD, Professor of Laboratory Medicine and Pathology, and Pamela McLean, PhD, Associate Professor of Neuroscience, both at the Mayo Clinic’s Florida Campus. Dr. Fitzpatrick will lead one of these projects, the goal of which is to characterize the nature and molecular structures of toxic protein aggregates found in Alzheimer’s, Parkinson’s and Lewy body dementia.
“To truly understand the link between proteins and disease, we must discover how they interact with each other and with the surrounding brain tissue,” said Dr. Fitzpatrick, who is also an assistant professor of biochemistry and molecular biophysics at Columbia’s Vagelos College of Physicians and Surgeons and member of the Taub Institute. “Cryo-EM’s power lies in its ability to reconstruct any protein at the level of individual atoms. This, combined with cryo-ET, which helps us understand how each protein behaves inside individual brain cells will, ultimately, enable us to learn how this behavior causes cells to die.”
Abnormally large deposits of the proteins called alpha-synuclein and amyloid-beta proteins are believed to be at the root of both Parkinson’s and Lewy body dementia. Difficult to diagnose definitively, these disorders are often seen in conjunction with each other. Symptoms include impaired cognition, memory loss, slow movement and tremors.
The main culprit behind Alzheimer’s is also abnormal protein deposits, in this case amyloid-beta and another protein called tau. As Alzheimer’s progresses, cells in the hippocampus (the brain’s memory center) degrade and die. Over time, patients experience greater difficulty recalling memories and navigating their everyday lives.
Despite decades of research on these disorders, there exists no treatment that can address the underlying biological causes of these diseases. This is where cryo-EM and cryo-ET can together prove transformational.
“One of the most imperative quests of our time is the understanding of brain function and the mechanisms of neurodegeneration,” said Zuckerman Institute Director and CEO Rui Costa, DVM, PhD. “This endeavor will allow us to have unprecedented insight into the 3D molecular structure of the aggregates that form in many neurodegenerative disorders, which ultimately can be critical for early diagnosis, prevention and treatment of these disorders.”
Cryo-EM is an innovative imaging technology that deduces a protein’s structure by firing beams of electrons at a protein that has been frozen in solution. Dr. Fitzpatrick and his team plan to use cryo-EM on post-mortem brain tissue extracted from patients diagnosed with Alzheimer’s, Parkinson’s and Lewy body dementia. He and his team will capture snapshots of protein complexes from different perspectives and then painstakingly reconstruct their three-dimensional structure.
Cryo-ET is an emerging technology that provides a high-resolution, visual map of a neuron’s interior. A longstanding scientific challenge has been finding a way to capture the activity happening inside neurons without destroying the neuron itself. Cryo-ET solves that problem.
In using cryo-ET to characterize the behavior of toxic protein aggregates inside whole, undissected neurons, the team will gather critical information on the origins of disease that would otherwise be impossible to learn. The researchers hope to pinpoint where the toxic proteins congregate inside neurons, as well as how these proteins interact with different parts of the neuron.
“For example, we hope to discover whether different proteins work in concert to spur disease progression,” said Dr. Fitzpatrick. “The combined use of cryo-EM and cryo-ET marks a tremendous leap forward from traditional, in vitro methods, which study the proteins’ behavior in a petri dish and thus do not replicate their natural environment.”
The findings gleaned from this research could play an important role in designing much-needed strategies to diagnose and treat these and other, related neurodegenerative disorders early, before symptoms arise.
“Dr. Fitzpatrick and his team are pioneers in driving leading-edge research to better understand what causes diseases like Alzheimer’s, Lewy body dementia, and Parkinson’s,” said Mike Shafer, president of materials and structural analysis at Thermo Fisher Scientific, which develops cryo-EM and cryo-ET technology. “That he received this grant to use cryo-EM and cryo-ET in combination to study these diseases in their natural environment is further proof that his work is of the utmost importance.”
###
Award Details:
Grant: U54 NS110435-01A1
Title: Synergistic Interaction of amyloid-beta and alpha-synuclein in Lewy body Dementia
Principal Investigators:
Dennis Dickson, MD, Professor of Laboratory Medicine and Pathology at the Mayo Clinic’s Florida Campus
Pamela McLean, PhD, Associate Professor of Neuroscience at the Mayo Clinic’s Florida Campus
Principal Investigator of Project 3: Anthony Fitzpatrick, PhD, Principal Investigator at Columbia’s Zuckerman Institute, Assistant Professor of Biochemistry and Molecular Biophysics at Columbia University Irving Medical Center, Member of the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain
Total award: $2,835,000 over five years
Columbia University’s Mortimer B. Zuckerman Mind Brain Behavior Institute brings together a group of world-class scientists and scholars to pursue the most urgent and exciting challenge of our time: understanding the brain and mind. A deeper understanding of the brain promises to transform human health and society. From effective treatments for disorders like Alzheimer’s, Parkinson’s, depression and autism to advances in fields as fundamental as computer science, economics, law, the arts and social policy, the potential for humanity is staggering. To learn more, visit: zuckermaninstitute.columbia.edu.
Media Contact
Anne Holden
[email protected]
Original Source
https:/