Penn study finds that as cells age, their ability to remove damaged proteins and structures declines, which could be a risk factor for neurodegenerative brain diseases
Credit: Andrea Stavoe, Penn Medicine; eLife
PHILADELPHIA – Cells dispose of harmful “trash” through autophagy, a normal and necessary process in which aggregated proteins and dysfunctional structures are handled. If any part of this fails, waste builds up inside cells, eventually killing them. According to a new study from the Perelman School of Medicine at the University of Pennsylvania, as cells age, their ability to shed harmful refuse declines. The findings suggest that the deterioration of autophagy in aged neurons–cells that never replicate and are as old as the bodies they inhabit–could be a risk factor for a suite of neurodegenerative diseases such as ALS (amyotrophic lateral sclerosis), Alzheimer’s, and Parkinson’s.
Using live-cell imaging of neurons from young and aged mice, Erika Holzbaur, PhD, a professor of Physiology, and first author Andrea Stavoe, PhD, a postdoctoral fellow in Holzbaur’s lab, published their study this week in eLife. The importance of autophagy was recognized in 2016 with the Nobel Prize in Physiology or Medicine.
“The current thinking among scientists is that a decline in autophagy makes neurons more vulnerable to genetic or environmental risks,” Holzbaur said. “What motivates our line of research is that most neurodegenerative diseases in which a deterioration of autophagy has been implicated, such as ALS, and Alzheimer’s, Huntington’s and Parkinson’s diseases, are also disorders of aging,”
At the start of autophagy, a component within the cell, called an autophagosome, engulfs misfolded proteins or damaged structures to be degraded, essentially sequestering this waste in a biological trash bag. The autophagosome then fuses with a second cellular structure, called a lysosome, that contains the enzymes needed to breakdown the garbage, allowing the components to be recycled and reused. This elegant waste-removal stream is what keeps neurons healthy, but in its absence, neurons eventually die due to the buildup of unattended refuse.
“Think city streets during a sanitation workers strike,” Stavoe said.
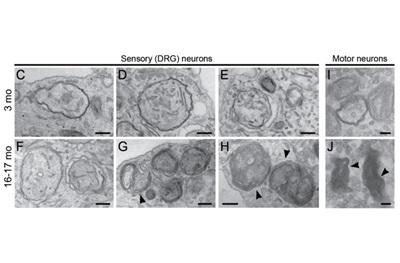
The team assessed rates of autophagy in mouse neurons during aging and identified a significant decrease in the number of autophagosomes produced, along with pronounced defects in the structure of autophagosomes produced by neurons from aged mice.
While early stages of autophagosome formation were unaffected, they found frequent stalling in their formation in aged mice, while the ones that did form were misshapen. These defects may allow the trash to accumulate at neuronal synapses. Stavoe notes that in other studies autophagosomes with misformed membranes have been observed in deceased human brain tissue from donors with neurodegenerative disease.
Importantly, turning on the protein WIPI2B in aged mice restores autophagosome formation in aged neurons, bringing the autophagy garbage-hauling process back online. This rescue is dependent on the level of activation of WIPI2B, providing insight into the biological regulation of autophagosome formation.
On the other hand, when researchers took WIPI2B out of young neurons, autophagosome formation stalled. “This stunning and complete rescue of autophagy using one protein suggests a novel therapeutic target for age-associated neurodegeneration,” Stavoe said.
###
Other co-authors are Pallavi Gopal from Yale University, and Andrea Gubas and Sharon Tooze, from The Francis Crick Institute, London.
This work was funded with an NIH Pathways to Independence Fellowship (K99 NS109286) and a Javits Award from NINDS (R37 NS060698).
TOPIC: Basic Science
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation’s first medical school) and the University of Pennsylvania Health System, which together form a $7.8 billion enterprise.
The Perelman School of Medicine has been ranked among the top medical schools in the United States for more than 20 years, according to U.S. News & World Report’s survey of research-oriented medical schools. The School is consistently among the nation’s top recipients of funding from the National Institutes of Health, with $425 million awarded in the 2018 fiscal year.
The University of Pennsylvania Health System’s patient care facilities include: the Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center–which are recognized as one of the nation’s top “Honor Roll” hospitals by U.S. News & World Report–Chester County Hospital; Lancaster General Health; Penn Medicine Princeton Health; and Pennsylvania Hospital, the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Home Care and Hospice Services, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is powered by a talented and dedicated workforce of more than 40,000 people. The organization also has alliances with top community health systems across both Southeastern Pennsylvania and Southern New Jersey, creating more options for patients no matter where they live.
Penn Medicine is committed to improving lives and health through a variety of community-based programs and activities. In fiscal year 2018, Penn Medicine provided more than $525 million to benefit our community.
Media Contact
Karen Kreeger
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




