Credit: [Credit: H. Liu et al., ScienceTranslational Medicine (2019)]
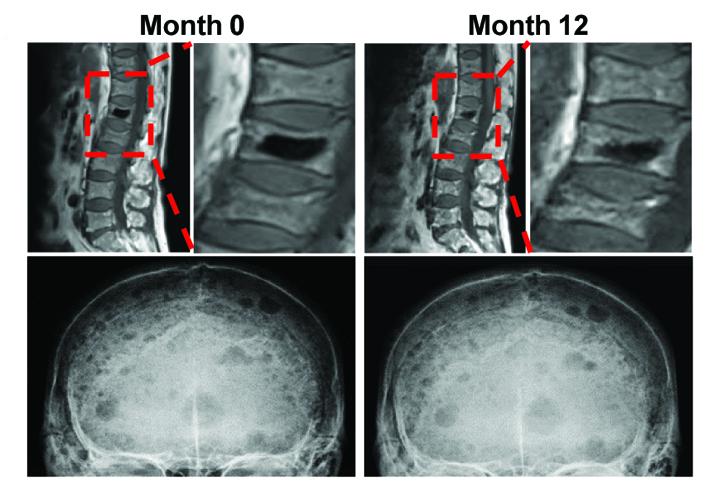
A study of samples from patients with multiple myeloma (MM) has demonstrated how “reprogrammed” fat cells contribute to long-lasting bone damage, even after the cancer has gone into remission. Targeting a molecular complex reduced the severity of damaging bone lesions in mice, hinting towards possible strategies for treating this common and debilitating complication of MM. This malignancy occurs when cancerous plasma cells accumulate in bone marrow and adversely impact the production of other blood cells. More than 80% of patients with MM also develop osteolytic lesions on their bones, which can cause severe pain and bone fractures. These lesions do not heal even if the underlying cancer is successfully treated, resulting in long-term deficits in bone healing and a lower quality of life. To understand why osteolytic lesions do not heal, Huan Liu and colleagues studied marrow samples from patients with active MM, patients in remission and healthy controls. They observed that the sites near osteolytic lesions harbored high amounts of bone marrow fat cells (or adipocytes). Adipocytes grown in culture alongside myeloma cells transitioned into a reprogrammed state where they released enzymes that suppressed bone formation and promoted bone breakdown. Further analysis showed myeloma cells converted adipocytes by activating a molecular complex called PRC2, which in turn repressed the activity of a receptor named PPARγ. Deactivating a component of PRC2 named EZH2 in adipocytes prevented their reprogramming and reduced the severity of bone lesions in a mouse model of MM remission, suggesting that restoring PPARγ activity could help heal bone lesions in patients.
###
Media Contact
Press Package Team
[email protected]
Related Journal Article
http://dx.




