Tumor therapy: Today, a new method for more accurate prediction of proton range is introduced into clinical routine for the first time worldwide
Credit: OncoRay / Christian Hahn, Nils Peters
Today (Tuesday, 23 April) marks the beginning of a new era in treatment planning at the University Proton Therapy Dresden of the University Hospital in Dresden (Germany): For the worldwide first time, a new approach increases the accuracy, safety and probably also the tolerability of proton therapy. The range prediction procedure was developed and extensively validated by medical physicists from the Dresden OncoRay Center, the Helmholtz-Zentrum Dresden-Rossendorf and the German Cancer Research Center in Heidelberg. Together with radiation oncologists, its clinical advantages were investigated.
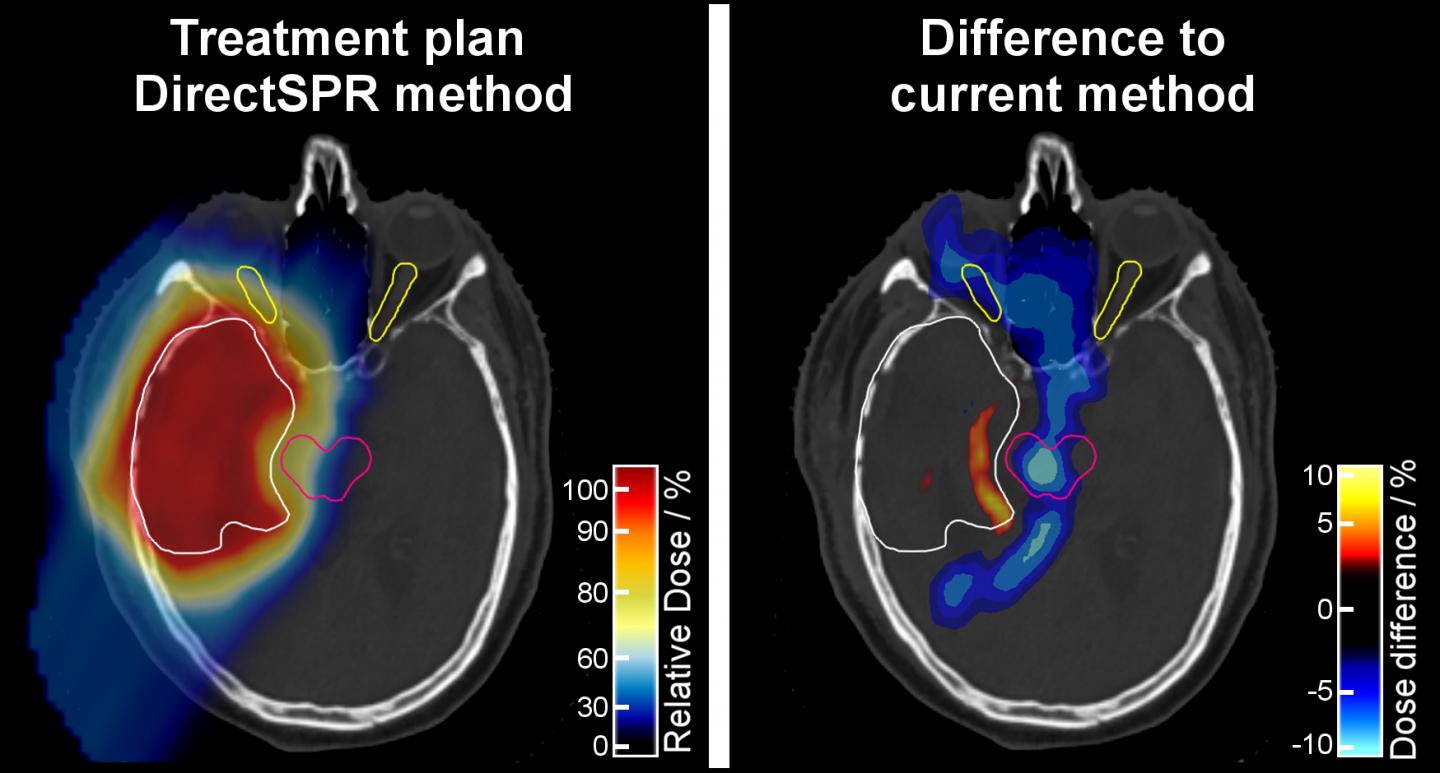
With the “DirectSPR” method, the proton range in human tissue can be predicted more accurately and on an individual patient basis. The Dresden researchers were able to demonstrate that the volume of irradiated healthy tissue surrounding the target volume can be reduced by 35 to 40 percent. The range accuracy, which has remained practically unchanged for more than 30 years, is thus for the first time significantly improved. The patients in Dresden will be the first benefitting from this.
Protons have particularly favorable properties for cancer therapy, as they release a large part of their energy exactly at the moment when they stop in the body. Thus, healthy tissue beyond the target can be better spared. However, medical physicists are faced with the challenge to determine the stopping behavior of the different tissue types, which the protons pass on their way to the tumor.
So far, mostly conventional computed tomography (CT) images have been used for this purpose. However, in human tissue the interaction processes of X-rays used for CT differ from those of protons used for therapy. Hence, the stopping behavior of protons and thus their total range can only be predicted with limited accuracy.
For this reason, a safety margin of healthy tissue surrounding the tumor needs to be irradiated with the same dose as the tumor to ensure that the tumor is completely irradiated. For prostate cancer, for example, this safety margin can be up to 12 millimeter. The range uncertainty concept in proton therapy has been standardly used since the 1980s. Also, the safety margins employed have been practically unchanged since then.
Dual-energy computed tomography (DECT), which was originally developed for the field of Radiology, provides medical physicists with more meaningful information for proton treatment planning. “So far, it was not possible to predict the range of the protons with high accuracy in patients,” explains Dr. Christian Richter, head of the research group High Precision Radiotherapy at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), which is one of the three founding partners of the hospital-based OncoRay Center, together with the University Hospital Dresden and the University of Technology Dresden. “Until now, we were lacking a technology for determining the individual parameters that define the stopping behavior of protons in different tissue types. With the DirectSPR approach, which is based on DECT, we are now able to do this. We can much better resolve differences in tissue composition between different patients, as well as in different tissues in the same patient, and take them into account during treatment planning. With this innovation, proton therapy becomes better tolerable, more accurate and also more tailor-made.”
From the dual-energy CT scan, two CT images, generated with different X-ray spectra, are available for further processing. They contain substantially more information on required tissue characteristics than with the previous standard method using conventional CT. Nevertheless, patients are not exposed to a higher X-ray dose compared to conventional CT. The only difference is that they are moved twice through the CT scanner.
The Dresden scientists were able to prove that the advantage of the DirectSPR method for range and dose calculation is not only theoretically existing, but also clinically highly relevant. “We have seen that the comprehensively validated DirectSPR approach, that was already proven to be more accurate, results in a proton range that is four to eight millimeters different from the previous standard method – for deep-seated tumors. At that moment, the high clinical relevance of this innovation was immediately clear to us,” says Prof. Esther Troost, Director of the Department of Radiotherapy and Radiation Oncology at the University Hospital Dresden and head of the research group Image-guided High Precision Radiotherapy at the HZDR.
“As the results of the studies were so unequivocal, we wanted our patients to benefit from the new approach as quickly as possible. The lower radiation volume, for example in the brain, allows an even better protection of important neurological structures, such as the brain stem or the optic nerves. Thereby, we reduce side effects and make proton therapy even more tolerable for each individual patient,” adds Prof. Mechthild Krause, also Director of the Department of Radiotherapy and Radiation Oncology at Dresden University Hospital and Director of the OncoRay Center. In concrete terms, this means a reduction of the safety margin for prostate treatments by around 35 percent, for brain tumor treatments even up to 40 percent.
As early as 2015, scientists from the German Cancer Research Center (DKFZ) in Heidelberg together with colleagues from Dresden developed and optimized the calculation algorithm. With DirectSPR, the relative electron density and the effective atomic number are determined individually in each voxel and are then used to determine the stopping behavior, the so-called stopping power, of the tissue. The abbreviation SPR stands for “Stopping Power Ratio” and expresses the energy loss of the protons per distance travelled relative to the energy loss in water.
An important prerequisite for the current clinical implementation of the DirectSPR approach was the worldwide-first routine application of DECT for clinical proton therapy planning at the University Hospital Dresden back in spring 2015. This enabled the scientists to retrospectively evaluate the DirectSPR approach using real patient data, which was previously only possible in phantoms. By doing so, they gained valuable information about the clinical relevance of the new method. The results have also convinced industry: Siemens Healthineers has been collaborating with the researchers in Dresden since 2016 to make the DirectSPR approach available as medical product to other proton therapy centers around the world. The product development will for the first time be presented at the international meeting of the European Society for Radiotherapy and Oncology (ESTRO) to be held from April 26 to 30, 2019 in Milan, Italy.
“As translational researchers we would certainly not have been successful without the excellent cooperation with the clinicians and clinical medical physicists at OncoRay,” says Christian Richter. He therefore regards the interdisciplinary and cross-institutional structure of the OncoRay Center with its three partner institutes – the University Hospital Carl Gustav Carus, the Helmholtz-Zentrum Dresden-Rossendorf and the Technische Universitaet Dresden – as an essential prerequisite for the rapid translation of research results into clinical application. The close cooperation with the Heidelberg Institute for Radiooncology at the National Center for Radiation Research in Oncology also makes it possible to bundle expertise and realize projects that would not have been implemented at one of the locations alone.
###
Media Contact
Christine Bohnet
[email protected]
Original Source
https:/




