More transition metals available to make ordered porous metallic oxide thin films
Credit: Tokyo Metropolitan University
Tokyo, Japan – Scientists from Tokyo Metropolitan University have developed a new method for making ordered arrays of nanoholes in metallic oxide thin films using a range of transition metals. The team used a template to pre-pattern metallic surfaces with an ordered array of dimples before applying electrochemistry to selectively grow an oxide layer with holes. The process makes a wider selection of ordered transition metal nanohole arrays available for new catalysis, filtration, and sensing applications.
A key challenge of nanotechnology is getting control over the structure of materials at the nanoscale. In the search for materials that are porous at this length scale, the field of electrochemistry offers a particularly elegant strategy: anodization using metallic electrodes, particularly aluminum and titanium, can be used to form ordered arrays of “nanoholes” in a metallic oxide layer. By getting the conditions right, these holes adopt highly ordered patterns, with tight control over their spacing and size. These ordered porous metallic oxide films are ideal for a wide range of industrial applications, such as filtration and efficient catalysis. But to get them out of the lab and into widespread use, production methods need to become more scalable and compatible with a wider range of materials.
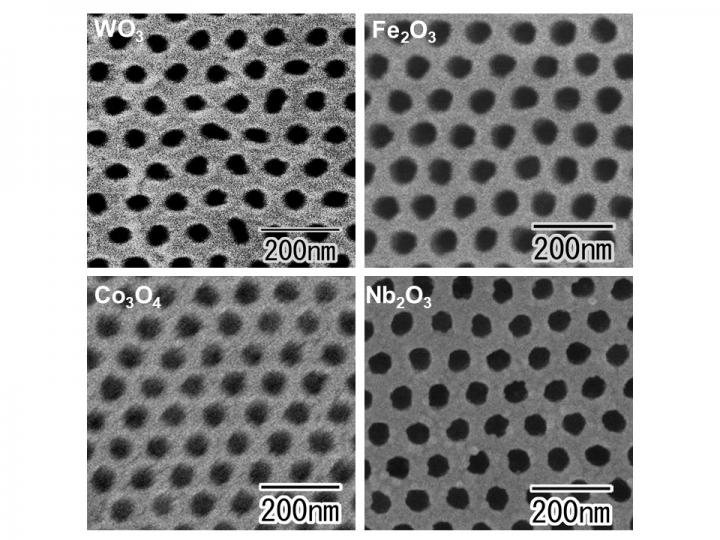
Enter a team led by Prof. Takashi Yanagishita of Tokyo Metropolitan University who have been pushing the boundaries of ordered nanohole array fabrication. In previous work, they developed a scalable method to make ordered nanohole arrays in aluminum oxide thin films. The team’s films could be made up to 70 mm in diameter, and easily detached from the substrates they are made on. Now, they have used these films to create similar patterns using a far wider range of transition metal oxides. By using the ordered nanoporous alumina as a mask, they used argon ion milling to etch ordered arrays of shallow dimples in the surfaces of various transition metals, including tungsten, iron, cobalt and niobium. Then by anodizing the dimpled surfaces, they found that thin metallic oxide layers formed with holes where the dimples were. Previous efforts had indeed made nanoscale holes in e.g. tungsten oxide films, but the holes were not ordered, with little control over their size or spacing, making this the first time ordered nanohole arrays have been made using these transition metal oxides. On top of that, by changing the properties of the mask, they directly demonstrated how they could easily tune the spacing between the holes, making their method applicable to a wide range of nanoporous patterns with different applications.
Exciting applications are waiting for these nanostructured films, including photocatalysis, sensing applications and solar cells. The team are confident that their new scalable, tunable method for making ordered nanohole arrays with freer choice of materials will help boost efforts to bring this exciting field of nanotechnology out of the lab, and into the wider world.
###
This work was supported by a JSPS KAKENHI Grant-in-Aid of Scientific Research, Grant Number JP20K05171.
Media Contact
Go Totsukawa
[email protected]
Related Journal Article
http://dx.




