Researchers at Keck School of Medicine of USC identify a novel target related to the blood-brain barrier and a potential therapy that offers hope for slowing progression of Alzheimer’s disease in people with the APOE4 gene
Like amyloid plaque, the genetic variant APOE4 has long been associated with Alzheimer’s disease, but still little is known about the role the gene plays in the disease process.
Now, a new study published in Nature Aging not only sheds light on how the gene may instigate a cascade of pathologies that contribute to Alzheimer’s disease, but also suggests a new treatment target that might help people who carry the APOE4 gene in early and late stages of the disease. Keck School of Medicine of USC researchers found that APOE4 is associated with the activation of an inflammatory protein that causes a breakdown in the blood-brain barrier which protects the brain.
This research builds on a recent USC study that revealed APOE4 triggers leaks in the blood-brain barrier in humans, which lets toxic substances from the blood stream into the brain, damaging brain cells and disrupting cognitive functions. This process causes memory problems in patients whether or not their brain shows signs of amyloid-β, the sticky plaque peptide considered a hallmark of the disease.
The latest findings also suggest a new potential treatment to slow down or prevent the cognitive decline associated with Alzheimer’s disease in patients with the APOE4 gene, independently of amyloid-β pathology.
“We’re further focusing on therapeutics targets in blood vessels that could bring innovative treatments to people suffering from Alzheimer’s disease, both early and late stages of the disease. Current findings in mouse models might be particularly promising for treating late stage disease in the presence of advanced amyloid-β pathology,” said Berislav Zlokovic, MD, PhD, director of the Zilkha Neurogenetic Institute at the Keck School of Medicine of USC.
The role of APOE4, pericytes and Cyclophilin A in Alzheimer’s disease
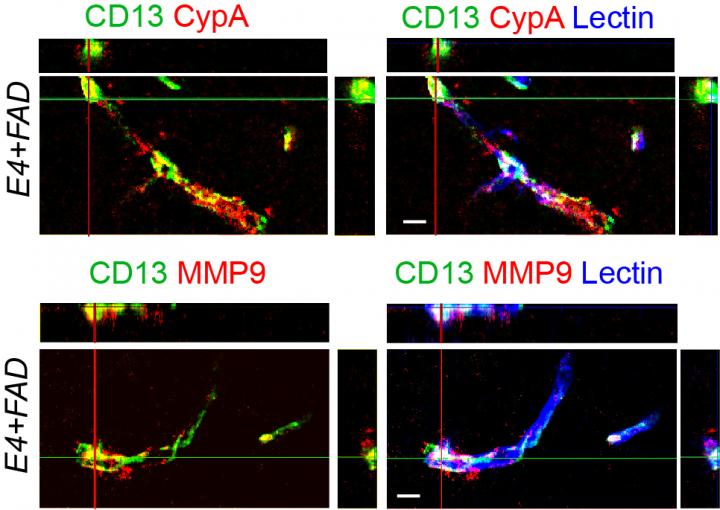
APOE4 has been shown to accelerate the blood-brain barrier breakdown by damaging pericytes, a layer of cells that strengthen and protect the brain capillaries which make up the blood-brain barrier. This breakdown is also associated with higher levels of Cyclophilin A, a pro-inflammatory protein, in the brain vessels of Alzheimer’s disease patients with the APOE4 gene.
In this study, USC researchers focused on Cyclophilin A in mice with the APOE4 gene, which carries a high risk for Alzheimer’s disease, and mice with the APOE3 gene, which carries an average risk for Alzheimer’s disease. Cyclophilin A is found in pericytes and controls how strong the blood vessels are in maintaining the integrity of the blood-brain barrier. In the APOE4 mice, researchers found Cyclophilin A caused an enzyme that degrades blood vessels in the blood-brain barrier — matrix metalloproteinase 9 (MMP9) — to become active. This did not happen in the APOE3 gene mice.
Researchers then tried treating APOE4 mice with an inhibitor known to suppress Cyclophilin A. The inhibitor not only improved integrity in the blood-brain barrier in APOE4 mice, but also prevented development of further neuron loss and behavioral deficits. Researchers observed that the APOE4 mice treated with the inhibitor did not exhibit behavioral deficits during daily activities. This suggests that treatment targeting this pathway might have the potential to also slow down the progression of vascular and neurodegenerative disorders in people with Alzheimer’s disease who have the APOE4 gene.
“So far there has been little hope for those in the late stage of the disease, which is very hard on patients and their loved ones,” said Zlokovic. “We are excited to further study the potential that interventions focused on blood-brain barrier repair and blood vessel strength, independent of amyloid pathology, could have on slowing down or stopping neurodegeneration and cognitive decline in advanced Alzheimer’s disease.”
The inhibitor used in this study to suppress the Cyclophilin A, Debio-025, has been used in humans to treat hepatitis C, suggesting this could be a potential treatment for cognitive impairment in APOE4 carriers that show Cyclophilin A-MMP9 pathway activity in early or late disease stages.
###
About the study
In addition to Zlokovic, other authors on the study include Axel Montagne, Angeliki Nikolakopoulou, Mikko Huuskonen, Abhay Sagare, Erica Lawson, Divna Lazic, Sanket Rege, Alexandra Grond, Edward Zuniga, Jacob Prince, Meghana Sagare, Ching Hsu and Russell Jacobs of the Department of Research Physiology & Neuroscience and Zilkha Neurogenetic Institute at the Keck School of Medicine of USC; Samuel Barnes of the Department of Radiology at Loma Linda University; and Mary LaDu of the Department of Anatomy and Cell Biology at the College of Medicine at the University of Illinois at Chicago.
This study was supported by the National Institutes of Health (R01NS034467, R01AG023084, R01AG039452 and 1R01NS100459), Cure Alzheimer’s Fund and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference number 16 CVD 05.
About Keck School of Medicine
Founded in 1885, the Keck School of Medicine of USC is one of the nation’s leading medical institutions, known for innovative patient care, scientific discovery, education, and community service. Medical and graduate students work closely with world-renowned faculty and receive hands-on training in one of the nation’s most diverse communities. They participate in cutting-edge research as they develop into tomorrow’s health leaders. With more than 900 resident physicians across 50 specialty and subspecialty programs, the Keck School is the largest educator of physicians practicing in Southern California.
Media Contact
Laura LeBlanc
[email protected]
Related Journal Article
http://dx.