Researchers at the University of Verona, in collaboration with the University of Glasgow and the Botton-Champalimaud Pancreatic Cancer Centre, are shedding light on an astonishing factor in pancreatic cancer that may revolutionize how we understand and treat this lethal disease. The study, which has recently garnered attention, highlights the significant role of extrachromosomal DNA (ecDNA) in enhancing the adaptability of pancreatic cancer cells, contributing to their survival, proliferation, and resistance to therapy. This research not only addresses the considerable challenges posed by pancreatic cancer but also opens new avenues for targeted treatments.
Pancreatic cancer has long been recognized as one of the deadliest malignancies, boasting a dismal five-year survival rate of only 13%. Its notorious reputation stems from its late diagnosis, often characterized as a “silent killer,” alongside its ability to swiftly adapt to therapeutic interventions. The current findings reveal that a substantial portion of the tumor cells leverage ecDNA to facilitate a rapid response to environmental pressures, essentially equipping them with a robust mechanism for survival and growth.
The researchers unearthed that many pancreatic cancer cells harbor multiple copies of vital oncogenes, such as MYC, situated on circular pieces of DNA that exist independently of chromosomes. This unique structure, ecDNA, which is capable of floating freely within the cell nucleus, enables these tumor cells to exhibit dynamic gene expression, thereby allowing them to modify their morphology and endure under unfavorable conditions. This discovery shows that pancreatic cancer cells employ ecDNA as a critical tool in their evolutionary toolkit, showcasing the complexity and adaptability inherent in these cancer cells.
Co-corresponding author Peter Bailey from the Botton-Champalimaud Centre elaborated on the findings, emphasizing the lethality of pancreatic cancer and its stealthy nature. The ability of these tumor cells to “shape-shift” under stress is underscored by the presence of ecDNA, marking a pivotal shift in understanding how some tumors can gain a survival advantage, especially in highly stressful environments. Notably, the researchers observed that this genomic feature was prevalent in pancreatic tumors, particularly for oncogenes like MYC, thus painting a broader picture of the tumor’s adaptable behavior.
The significant increase in variability of MYC copy number was noted particularly when MYC resided on ecDNA. The researchers found that certain cells possessed numerous additional copies of MYC, dramatically boosting their growth potential under specific conditions. This phenomenon illustrates a ‘bet-hedging’ strategy, where diverse populations within the tumor carry differing amounts of MYC. Some cells thrive under high MYC expression, while others with lower levels may fare better in alternative environments. This intricate balancing act among the populations encapsulates the profound intratumor heterogeneity that defines pancreatic cancer.
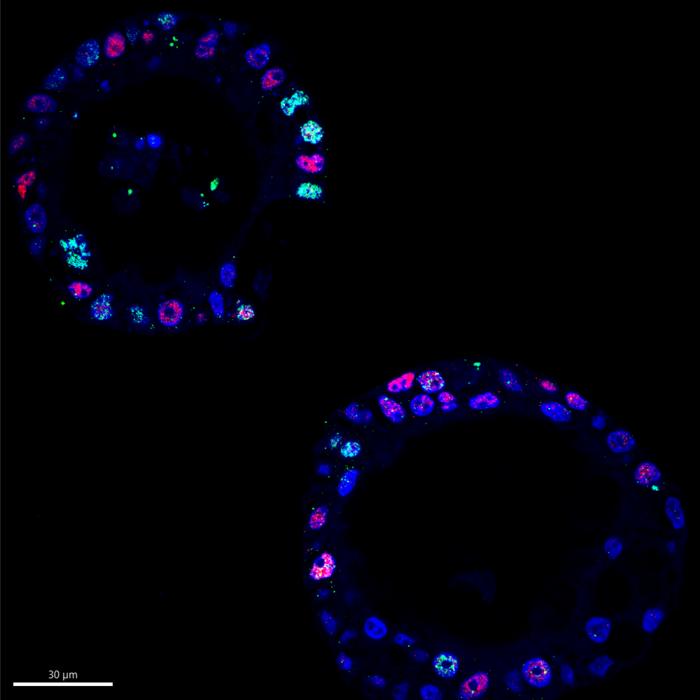
A pivotal methodology employed in this study involved the use of organoids—three-dimensional miniaturized models of pancreatic tumors derived directly from patients with early-stage pancreatic cancer. These organoids authentically replicate the genetic landscape of the original tumors, providing an invaluable platform for cancer research. Unlike traditional approaches that manipulate ecDNA artificially, studying these organoids enables researchers to observe genuine ecDNA variations and their effects within the tumor environment.
In exploring how ecDNA contributes to cancer cell adaptation, the researchers subjected the patient-derived organoids to a controlled environment where they removed critical growth signals, such as WNT factors. This experimental design illuminated the ability of organoids carrying MYC on ecDNA to become more autonomous, reducing their reliance on external growth signals for survival. The findings were particularly significant, as they reveal the adaptability of cells that harbor ecDNA—bolstering their self-sufficiency amidst hostile conditions.
As the study progressed, it became evident that high MYC levels correlated with substantial changes in tumor cell morphology and behavior. Increased ecDNA levels caused the cells to adopt more aggressive, solid structures, often at the expense of their gland-like organization. These observations led researchers to suggest that the emergence of ecDNA allows for rapid genomic adaptations, prompting cells to respond effectively to fluctuating environmental pressures by adjusting their morphologies and functional dynamics.
In a remarkable finding, the researchers pointed out that ecDNA-endowed copies of MYC can appear and disappear with astonishing rapidity depending on external stimuli. For instance, in circumstances of acute growth factor deprivation, those cancer cells could significantly ramp up MYC expression, securing their survival advantage. Conversely, under less stressful conditions, these cells might selectively shed some of the excess ecDNA to minimize the potential risks associated with high MYC levels, which can lead to DNA damage.
The implications of these findings on the therapeutic landscape for pancreatic cancer could be vast. Although ecDNA appeared in approximately 15% of the samples analyzed in this study, this subset may represent a particularly aggressive faction of tumors that possess heightened resistance to established therapies. Consequently, identifying or targeting ecDNA could herald new opportunities for treatment strategies aimed at enhancing patient outcomes.
A therapeutic approach could involve pushing these cancer cells to overload on MYC expression, thereby inducing an unmanageable level of DNA damage. Alternatively, blocking the molecular pathways that sustain these ecDNA structures might lead to their degradation, forcing tumor cells to lose their genetic advantages. However, researchers caution that these strategies remain preliminary at this stage, as the dual nature of ecDNA poses significant challenges; while it facilitates rapid adaptation, it comes with the metabolic cost of maintaining extra genetic material.
The research broadens our comprehension of genomic plasticity, dispelling the notion that our genomes are static and immutable. The discovery that WNT signaling can directly influence DNA architecture was unexpected and highlights the dynamism with which tumors can evolve and adapt to their microenvironments. The implications are particularly crucial given the projected increase in pancreatic cancer incidence in upcoming years, signaling an urgent need for innovative interventions that target these genetic features to enhance treatment susceptibility.
As we delve deeper into the nature of pancreatic cancer, the multifaceted role of ecDNA emerges as a formidable topic that merits further exploration. The potential for targeted therapies that exploit the vulnerabilities linked to ecDNA could pave the way for advancements in treatment protocols. Ultimately, continued research in this area will be instrumental in uncovering new strategies to combat a disease that has historically posed significant therapeutic challenges.
This research underscores the importance of continuing to investigate complex genetic features that influence cancer behavior and treatment outcomes. By understanding the nuanced dynamics of ecDNA and its implications in intratumor heterogeneity, scientists may unlock new pathways for intervention, ultimately improving the prognosis for patients with one of the most formidable adversaries in oncology.
Subject of Research: Pancreatic cancer and its relationship with extrachromosomal DNA.
Article Title: MYC ecDNA promotes intratumor heterogeneity and plasticity in PDAC.
News Publication Date: 12-Mar-2025.
Web References: Link to Journal
References: Nature.
Image Credits: Vinzenzo Corbo Lab.
Keywords: pancreatic cancer, extrachromosomal DNA, MYC, tumor heterogeneity, therapy resistance, genomic plasticity.
Tags: adaptability of cancer cellschallenges in treating pancreatic cancerextrachromosomal DNA in pancreatic cancerimproving pancreatic cancer survival ratesinnovative pancreatic cancer therapieslate diagnosis of pancreatic canceroncogenes in pancreatic tumorsresistance to cancer therapyrole of ecDNA in cancersurvival mechanisms of pancreatic cancertargeted treatments for pancreatic cancerUniversity of Verona cancer research