Credit: Chinese Journal of Catalysis
Using intermittent electric energy to convert excessive CO2 into C2 products, such as ethylene and ethanol, is an effective strategy to mitigate the greenhouse effect. Copper (Cu) is the only single metal catalyst which can converts CO2 into C2 products by electrochemical method, but with undesirable selectivity of C2 product. Therefore, how to improve the conversion efficiency of Cu-based catalysts for reducing CO2 to C2 product has attracted great attention.
Recently, a research team led by Prof. Min Liu from Central South University, China designed a Cu-Pd bimetallic electrocatalyst possessing CuPd(100) interface which can lower the energy barrier of C2 product generation. The electrocatalyst was obtained through using an in-situ growth method based on thermal reduction to afford Pd nanoparticles as nucleated seeds. The results were published in Chinese Journal of Catalysis.
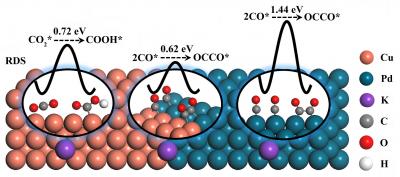
Generally, there are two limiting factors for achieving the electroreduction of CO2 to C2 products, namely the amount of CO* intermediate (* indicates the intermediate is adsorbed on the surface of the catalyst) and the C-C coupling step (generally two CO* coupling). For Cu catalysts, the energy barrier of the C-C coupling step is relatively low. However, the CO2 adsorption and CO2* hydrogenation ability of Cu are unfavorable, resulting in insufficient amount of CO* involved in subsequent C-C coupling step. Palladium (Pd) is an efficient catalyst that exhibited strong CO2 adsorption and ultrafast reaction kinetics for CO* formation. However, CO* poisoning on the Pd surface makes it unsuitable for generating C2 products. To take full advantage of both Cu (low energy barrier of C-C coupling) and Pd (ultrafast kinetics for CO* formation), the assembly of a CuPd bimetallic catalyst was envisaged as a potential method for optimizing the efficiency of C2 product formation.
The density functional theory (DFT) calculation shows that the CuPd (100) interface enhanced the adsorption of CO2 and reduced the energy barrier of CO2* hydrogenation step, thus sufficient CO* participated in the C-C coupling reaction. In addition, the energy barrier of rate-determining step for C2 product generation on CuPd (100) interface is 0.61 eV, which is lower than that on Cu(100) surface (0.72 eV).
Then the target CuPd (100) interface catalyst was prepared by a simple wet chemical method and proved by different characterization methods. The temperature programmed desorption and gas sensor experiment results proved the enhanced CO2 adsorption and CO2* hydrogenation ability on CuPd(100) interface, respectively. As a result, the CuPd(100) interface catalyst exhibited a C2 Faradaic efficiency of 50.3%, which was 2.1 times higher than that of Cu catalyst (23.6%) at -1.4 VRHE in 0.1 M KHCO3. This work provides a reference for the rational design of Cu-based electrocatalyst for CO2 electroreduction by adjusting the intermediate reaction energy barrier.
###
This work was supported by the Natural Science Foundation of China (Grant Nos. 21872174, 22002189, 51673217 and U1932148), the International Science and Technology Cooperation Program (Grant No. 2017YFE0127800 and 2018YFE0203402), the Hunan Provincial Science and Technology Program (2017XK2026), the Hunan Provincial Natural Science Foundation (2020JJ2041and 2020JJ5691), the Hunan Provincial Science and Technology Plan Project (Grant No. 2017TP1001).
About the Journal
Chinese Journal of Catalysis is co-sponsored by Dalian Institute of Chemical Physics, Chinese Academy of Sciences and Chinese Chemical Society, and it is currently published by Elsevier group. This monthly journal publishes in English timely contributions of original and rigorously reviewed manuscripts covering all areas of catalysis. The journal publishes Reviews, Accounts, Communications, Articles, Highlights, Perspectives, and Viewpoints of highly scientific values that help understanding and defining of new concepts in both fundamental issues and practical applications of catalysis. Chinese Journal of Catalysis ranks among the top six journals in Applied Chemistry with a current SCI impact factor of 6.146. The Editors-in-Chief are Profs. Can Li and Tao Zhang.
At Elsevier http://www.
Manuscript submission https:/
Media Contact
Fan He
[email protected]
Related Journal Article
http://dx.




