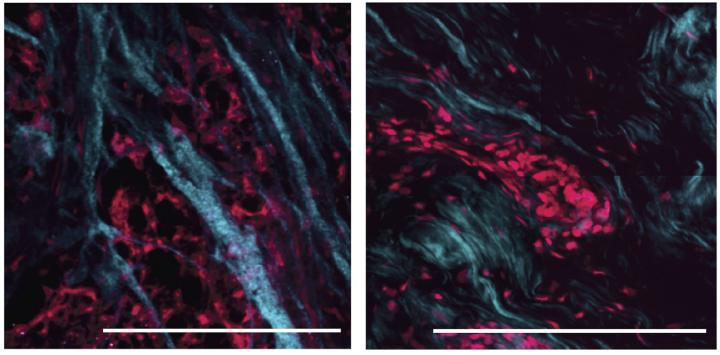
Credit: : ©2021 Drain et al. Originally published in Journal of Experimental Medicine. https://doi.org/10.1084/jem.20191360
Researchers at the University of California, San Francisco, have discovered that aggressive, triple-negative breast cancers (TNBCs) can evade treatment by reorganizing and softening the collagen matrix that surrounds the cancer cells. The study, which will be published April 2 in the Journal of Experimental Medicine (JEM), shows that the softer matrix activates a signaling pathway that promotes the cancer cells’ survival, and suggests that targeting this pathway could enhance the effectiveness of chemo- and radiotherapy in TNBC patients.
TNBC is an aggressive type of breast cancer with worse survival rates than other forms of the disease. Because TNBC cells lack the HER2 signaling receptor and the estrogen and progesterone hormone receptors, they cannot be eliminated by drugs that specifically target these proteins. Instead, TNBC patients are normally treated with a combination of surgery and chemotherapy/radiation, but these treatments are often unable to completely eliminate the tumor, leading to local recurrence and metastasis to other tissues.
“Currently, there are no reliable methods to identify patients that are likely to respond well and those that are likely to be resistant to treatment,” says Valerie M. Weaver, a professor and Director of the Center for Bioengineering and Tissue Regeneration in the Department of Surgery at UCSF. “There is an urgent need for a deeper understanding of the biological basis for therapeutic response in TNBC that will inform treatment decisions and the development of effective strategies to overcome therapy resistance.”
One factor that could influence the response to therapy is the meshwork of collagen and other proteins that surrounds the cancer cells within tumors. This extracellular matrix can activate various signaling pathways within cells that determine whether or not cells die when they are exposed to stress.
Weaver and colleagues, including Catherine C. Park, professor and Chair of the Department of Radiation Oncology at UCSF, and graduate student Allison P. Drain, analyzed the extracellular matrix in tumor samples taken from TNBC patients. In untreated TNBC, collagen proteins were aligned into long, linear fibers, creating a matrix that is much stiffer than normal, healthy breast tissue. But in TNBC treated with chemotherapy, the collagen fibers in the remaining tumor were reorganized to form a much softer matrix.
“This raised the intriguing possibility that the softened, remodeled extracellular matrix might be causally linked to the pathogenesis of the treatment-resistant, residual tumor tissue,” Weaver says.
The researchers found that tumors grown in the lab or in mice were more resistant to both radiation and the chemotherapy agent paclitaxel when they were surrounded by a softer extracellular matrix. Cancer cells grown in a stiff environment activate a signaling protein called JNK that makes the cells more likely to die in response to stress. In contrast, cancer cells surrounded by a softer matrix activate a protein called NF-kB that counteracts JNK and promotes cell survival. Accordingly, treating mice with a drug that inhibits NF-kB improved their therapeutic response, significantly slowing tumor growth when combined with radiotherapy.
Weaver and colleagues found that TNBC patients whose tumors had high levels of NF-kB activity showed higher rates of treatment resistance. NF-kB activity was also elevated in remodeled, softened tumors that remained after treatment.
“Therapies designed to modulate stiffness-dependent NF-κB or JNK activity might therefore improve the treatment response of TNBCs and enhance the long-term survival of these patients,” Weaver says. “However, further efforts will be needed to clarify the nature of the mechanisms that mediate treatment resistance and develop effective, selective, and safe therapeutics.”
###
Drain et al. 2021. J. Exp. Med.
https:/
About Journal of Experimental Medicine
Journal of Experimental Medicine (JEM) publishes peer-reviewed research on immunology, cancer biology, stem cell biology, microbial pathogenesis, vascular biology, and neurobiology. All editorial decisions on research manuscripts are made through collaborative consultation between professional scientific editors and the academic editorial board. Established in 1896, JEM is published by Rockefeller University Press, a department of The Rockefeller University in New York. For more information, visit jem.org.
Visit our Newsroom, and sign up for a weekly preview of articles to be published. Embargoed media alerts are for journalists only.
Follow JEM on Twitter at @JExpMed and @RockUPress.
Media Contact
Ben Short
[email protected]
Original Source
https:/
Related Journal Article
http://dx.



