Osaka University researchers show that salt crystals can precipitate at low concentrations owing to local density fluctuation and their repeated precipitation-dissolution behavior promotes aggregation of Amyloid-β peptides implicated in Alzheimer’s
Credit: Osaka University
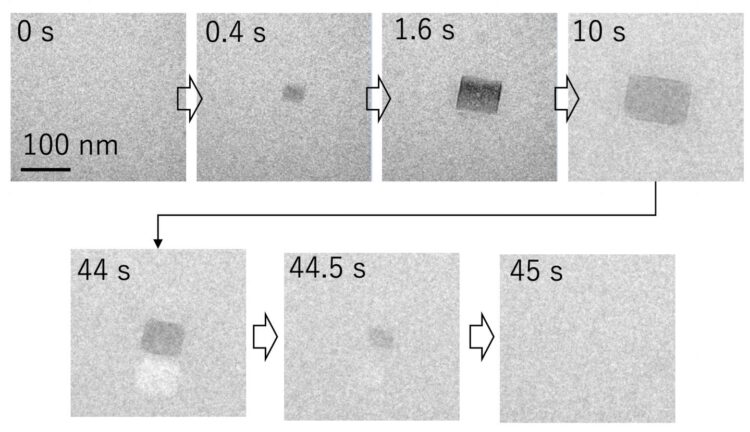
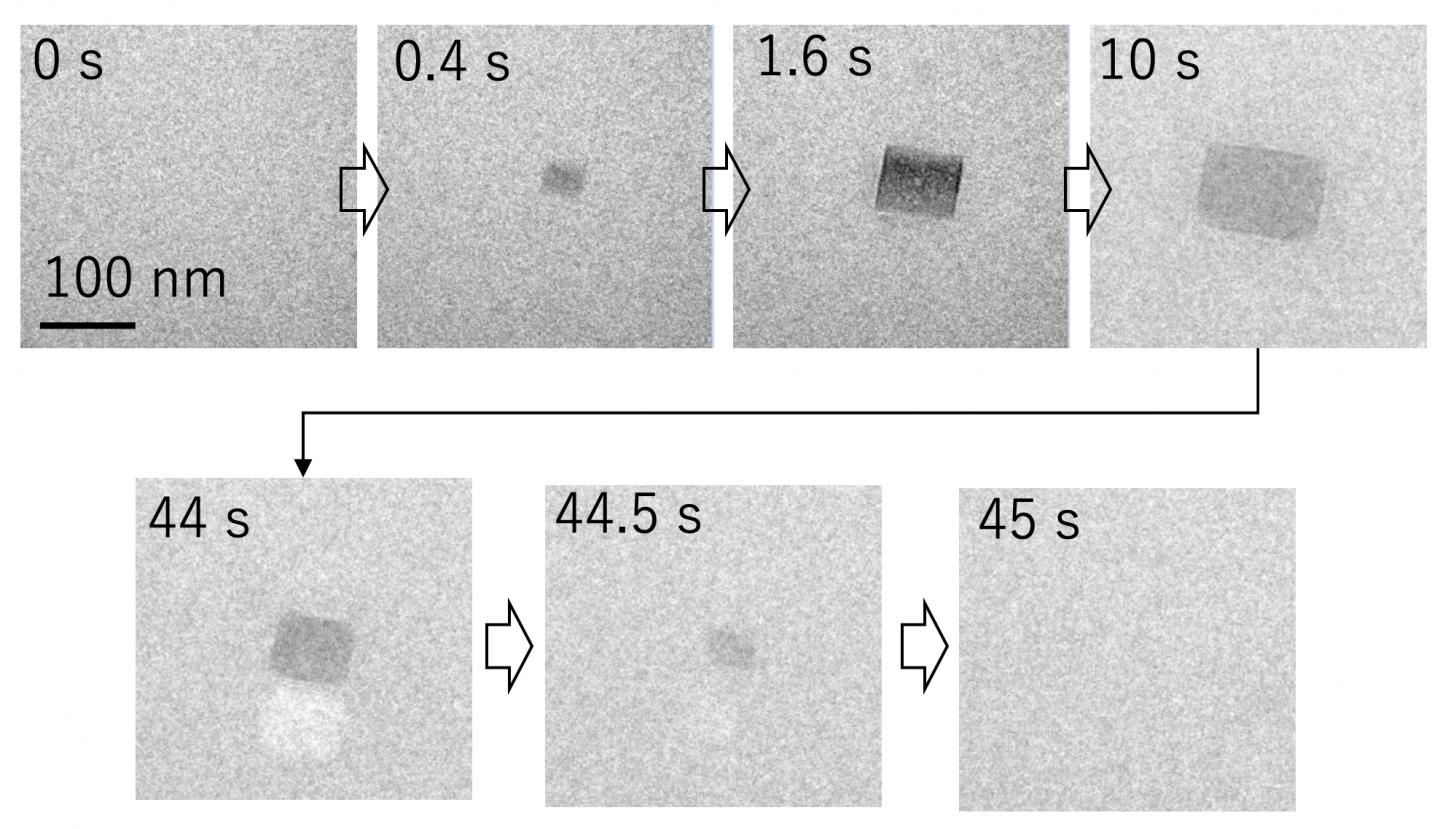
Osaka, Japan – Alzheimer’s disease is the leading cause of dementia worldwide and a major cause of disability. Now, researchers at Osaka University and Hokkaido University have shown that repeated precipitation-dissolution events of salt crystals do occur even at low salt concentrations in nanoscales, and that it can accelerate the aggregation of the neurotoxic amyloid-β peptides implicated in its pathogenesis.
The human brain comprises around 86 billion neurons, roughly as many grains of sand as in a large dump truck. These neurons juggle electrochemical information as signals among the brain, muscles and organs to orchestrate the symphony of life from survival to self-awareness. Alzheimer’s disease disrupts this complex neuronal networking, causing functional disability and cell death. As yet uncurable, available treatments are symptomatic, supportive, or palliative; a breakthrough in understanding its pathogenesis may brighten the prospects for medication, diagnosis and prevention.
The role of amyloid in Alzheimer’s disease has long been recognized. Amyloid-β peptides are derived from amyloid precursor protein and they self-assemble into sizes ranging from low-molecular-weight aggregates and larger oligomers to amyloid fibrils. These last are known to be neurotoxic but recent research suggests that oligomeric disordered aggregates are also toxic, possibly even more than fibrils.
“Fibril aggregation begins with nucleation followed by an elongation stage,” explains Kichitaro Nakajima, lead author of this study. “Until now, the early stages of oligomer evolution have been difficult to study because of their morphologic variability, the timeframe for nucleation, and the lack of a suitable fluorescent assay.”
Using liquid-state transmission electron microscopy, the researchers analyzed the aggregation of protein molecules, acquiring time-resolved nanoscale images and electron diffraction patterns. “Remarkably, we discovered that a salt crystal can precipitate even at a concentration well below its solubility due to local density fluctuation, and its rapid dissolution accelerates the aggregation reaction of amyloid-β peptides,” says Professor Hirotsugu Ogi, the corresponding author. “This formation of temporary salt crystals provides a mechanism whereby proteins adhere to the surface of the crystal; as it dissolves, the interface shrinks, condensing the proteins at the vanishing point. This phenomenon resembles the aggregation acceleration by ultrasonic cavitation bubble. Proteins are attached on the bubble surface during the expansion phase, and they are highly condensed by the subsequent bubble collapses by the positive pressure of ultrasonic wave at its center. This is the artificial catalytic effect. Thus, in an autocatalytic-like nanoscopic aggregation mechanism, salt dissolution accelerates the aggregation reaction, and the aggregate itself can promote salt nucleation.”
Ogi explains the implications of their results: “The aggregation of amyloid-β peptides is slow and this has been a hindrance to pharmaceutical research. Establishing an effective acceleration method will help clarify their structural evolution from monomer to fibril. This knowledge is key to understanding the pathogenesis of Alzheimer’s disease.”
###
The article, “Time-resolved observation of evolution of amyloid-β oligomer with temporary salt crystals,” was published in The Journal of Physical Chemistry Letters at DOI: https:/
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan’s most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.