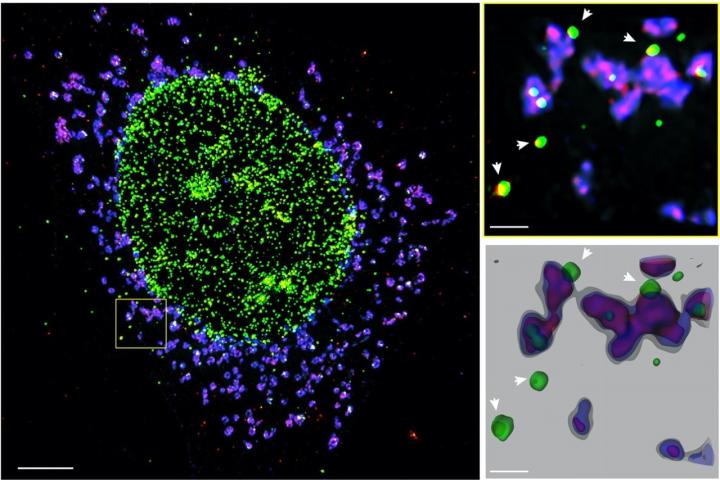
Credit: Image courtesy of the Walter and Eliza Hall Institute of Medical Research
Melbourne researchers are working towards a potential treatment to slow the progression of motor neuron disease (MND), offering hope to people with this debilitating and incurable illness.
The research team have uncovered how inflammation in MND is triggered. Pinpointing the molecules involved in this pathway could be a first step towards a new treatment for MND.
They found that by blocking an immune sensor called STING, they could dramatically prevent inflammation from MND patient cells, paving the way for a new class of drugs to be developed for people with neurodegenerative disorders, such as MND.
The discovery, published today in Cell, was led by Walter and Eliza Hall Institute researchers Associate Professor Seth Masters and Dr Alan Yu, with colleagues from the University of Melbourne and Hudson Institute.
At a glance
- Researchers have uncovered the primary immune pathway that is activated by a protein that accumulates in patients with MND
- By blocking the STING immune sensor, researchers were able to prevent inflammation from patient motor neurons, and thus promote motor neuron survival
- The discovery is a first step towards a treatment therapy for MND
Halting the inflammatory response
MND is an incurable condition in which the nerve cells controlling the muscles that enable us to move, speak, swallow and breathe, fail to work. One in 10,000 Australians will be diagnosed with MND in their lifetime and the average life expectancy from diagnosis is just two years.
Most people suffering from MND have an accumulation of a protein called TDP-43 within cells of the central nervous system. This build-up is associated with an inflammatory response that precedes major symptoms of MND.
Institute researchers investigated how the disease-causing inflammation is triggered in MND, said Associate Professor Masters. “This unexpectedly identified that an immune sensor called STING is activated downstream of TDP-43. Fortuitously, our team had already studied the role of STING in other inflammatory diseases and are now working out how to block it.”
The team then used new inhibitors – drug-like compounds – to block different components of this inflammatory pathway.
“Using cells from patients with MND that we can turn into motor neurons in a dish, we showed that blocking STING dramatically prevented inflammation and kept the cells alive longer. This is an exciting first step before taking these inhibitors into the clinic for treatment for MND.
Vital first step towards a treatment
Associate Professor Masters said his research had also established activation of STING in people who had passed away due to MND.
“We are now aiming to validate a biomarker of the pathway earlier in the disease progression. Once this neuroinflammatory biomarker is validated, we will better understand which patients will benefit the most from treatments targeting the pathway,” he said.
“With this knowledge, there is the potential to develop a treatment for patients with MND.
“Interestingly, our preclinical models suggest that although the anti-inflammatory drugs that inhibit STING did not prevent disease onset, they did slow the degenerative progression of disease.”
Hope for people with MND and other neurodegenerative disorders
Associate Professor Masters said this discovery offered hope for people diagnosed with the debilitating condition.
“We are hopeful this research could lead to a treatment for people with established MND, who currently have very few treatment options and a life expectancy post diagnosis of just two to five years,” he said.
“While it isn’t a cure, we hope it might extend life expectancy and dramatically improve the quality of life for people diagnosed with MND.”
Associate Professor Masters said a future treatment might also be effective in slowing the progression of other neurodegenerative disorders.
“We are hoping to develop a new class of drugs that would act as STING inhibitors to stop the progression of neurodegenerative disorders, such as MND, Frontotemporal Dementia and Parkinson’s disease.”
###
The research was funded by the Australian National Health and Medical Research Council, veski, HHMI-Wellcome Trust, the Sylvia and Charles Viertel Foundation (SLM), the Australian Research Council, Fellowship Fonds de recherche du Québec-Santé, the WEHI Centenary Fellowship and Ormond College’s Thwaites Gutch Fellowship in Physiology, the Motor Neurone Disease Research Institute of Australia, the Australian Phenomics Network, the Ian Potter Centre for Genomics and Personalized Medicine and the Victorian Government.
Media Contact
Samantha Robin
[email protected]
Related Journal Article
http://dx.




