Anion exchange membrane fuel cells (AEMFCs) have gained attention in the process of fuel cell development because they operate in alkaline environments, the redox reaction rate at the electrodes is faster, and non-precious metal catalysts such as Ni, Co, and Ag can be used, which reduces the cost of fuel cells. However, the mobility of OH– is only 56.97% of that of H+ under the same conditions, and its stability is poor, so improving the ionic conductivity and mechanical properties of anion exchange membranes (AEM) is the key to the commercialization of AEMFC. Recently, a team of scientists has constructed a poly (p-terphenyl isatin) anion exchange membrane with quaternary ammonium and piperidine cations synergistic functionalization that provides excellent mechanical properties and OH-ion conductivity for alkaline anion membrane fuel cells. Their work is published in the journal Industrial Chemistry & Materials on 14 September 2023.
Credit: Zhe Wang, Changchun University of Technology, Changchun, China.
Anion exchange membrane fuel cells (AEMFCs) have gained attention in the process of fuel cell development because they operate in alkaline environments, the redox reaction rate at the electrodes is faster, and non-precious metal catalysts such as Ni, Co, and Ag can be used, which reduces the cost of fuel cells. However, the mobility of OH– is only 56.97% of that of H+ under the same conditions, and its stability is poor, so improving the ionic conductivity and mechanical properties of anion exchange membranes (AEM) is the key to the commercialization of AEMFC. Recently, a team of scientists has constructed a poly (p-terphenyl isatin) anion exchange membrane with quaternary ammonium and piperidine cations synergistic functionalization that provides excellent mechanical properties and OH-ion conductivity for alkaline anion membrane fuel cells. Their work is published in the journal Industrial Chemistry & Materials on 14 September 2023.
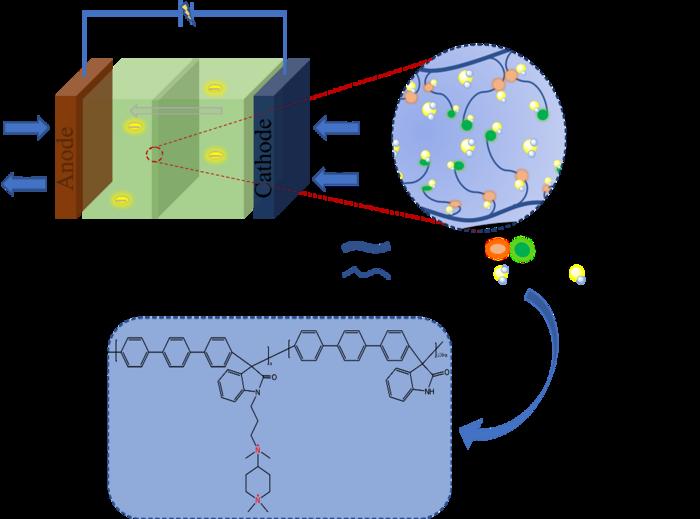
AEM can act on alkaline fuel cells and in the form of transported OH–, greatly reducing production costs. This is a boost to the general application of clean energy, but the biggest challenge is the “trade-off” between the ionic conductivity and stability of anion exchange membranes. “Anion exchange membrane is an important component in fuel cells, and the performance of the membrane profoundly affects the performance and development of fuel cells. How to make a breakthrough in the performance of anion exchange membrane and how to achieve high power density are the issues that our research team has been exploring,” explains Zhe Wang, a professor at the Changchun University of Technology.
Most of the conventional anion exchange membrane polymer backbones contain ether bonds, whose subsequent reactions are not only toxic and harmful, but also highly susceptible to attack and degradation during OH– transport. Not only that, the presence of hydrophilic groups in the polymers containing ether-bonded structures may cause the AEM to dissolve too much, leading to a sharp decrease in its stability.
The researchers used a polymer backbone without ether bonds to fundamentally solve the problems associated with conventional backbones. The quaternary ammonium cation and piperidine cation in the anion exchange membrane work in concert to construct a tighter ion-transport channel, which reduces the activation energy required to transport OH– and facilitates the flow of OH– within the membrane. The piperidinium cation also has superior alkaline stability due to its cyclic conformation, while the alkali stability of a series of anion exchange membranes in the paper reached 800 h on the basis of the ether-bond-free backbone.
In addition to these aspects, the researchers also confirmed that the mechanical properties of poly (p-terphenyl isatin) series membranes are in an excellent state. The tensile strength of the pure membranes reaches more than 60 MPa, which provides a stable foundation for further functionalization of subsequent anion exchange membranes.
Looking ahead, the team hopes that their work will provide new ideas for the further development of anion exchange membranes. “Our next step will be to focus on targeting battery power density improvement to achieve the ultimate goal of industrialized application of anion-exchange membranes,” said Wang.
The research team includes Yiman Gu, Yanchao Zhang, Zhe Wang*, Di Liu, Yan Wang, Tianming Dong, Song Wang, Zhanyu Li, Jingyi Wu and Yijia Lei from the Changchun University of Technology.
This research is funded by the Natural Science Foundation of China, Jilin Provincial Science & Technology Department.
Industrial Chemistry & Materials is a peer-reviewed interdisciplinary academic journal published by Royal Society of Chemistry (RSC) with APCs currently waived. Icm publishes significant innovative research and major technological breakthroughs in all aspects of industrial chemistry and materials, especially the important innovation of the low-carbon chemical industry, energy, and functional materials.
Journal
Industrial Chemistry and Materials
DOI
10.1039/D3IM00077J
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Synergistic functionalization of poly(p-terphenyl isatin) anion exchange membrane with quaternary ammonium and piperidine cations for fuel cells
Article Publication Date
14-Sep-2023




