A research group at São Paulo State University (UNESP) analyzed how Candida albicans fungi and Staphylococcus aureus bacteria influence gene expression and tumor cell survival
Credit: FOAr-UNESP
An in vitro study conducted by a group of researchers at São Paulo State University (UNESP) in Araraquara, Brazil, shows how fungi and bacteria can activate genes associated with head and neck tumors, as the metabolism of biofilms (communities in which these microorganisms self-organize in a structured and coordinated manner) stimulate tumor cells by favoring the cell signaling pathways required for tumor development and resistance to treatment. The findings include entirely novel information on the links between microbial biofilms and cell behavior in head and neck cancer.
The researchers discovered that metabolites secreted by biofilms, termed the secretome, can modulate the expression of proto-oncogenes and cell cycle genes associated with tumor cell growth and survival. Their analysis of gene expression focused on two signaling pathways (EGFR/RAS/RAF/MEK/ERK and EGFR/PI3K/AKT/mTOR) that play a key role in tumor cell proliferation, differentiation, and survival. Alterations to gene expression in these pathways are highly prevalent in various types of tumor.
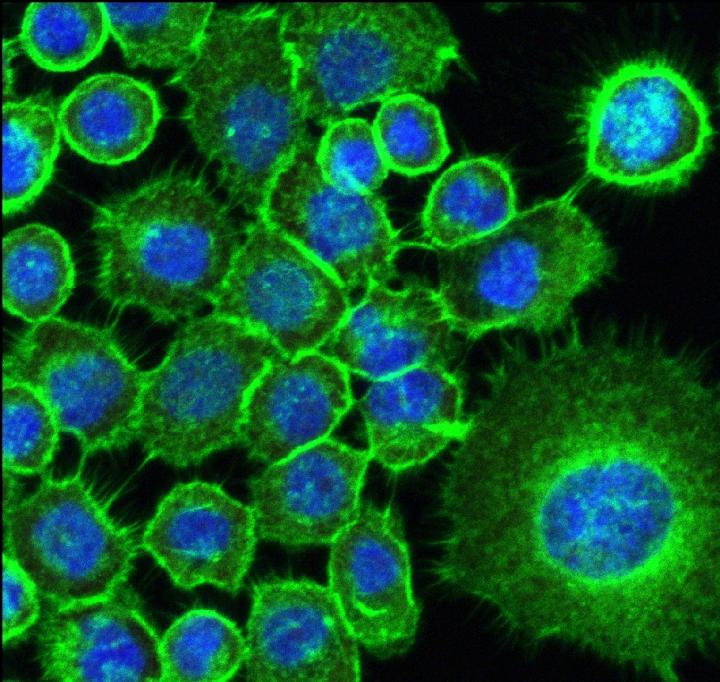
The researchers analyzed head and neck and oral cavity squamous carcinoma cells. Squamous cell carcinoma is the most common type of mouth cancer, which produces functional and aesthetic changes that degrade the patient’s quality of life.
The cells were challenged via stimulation by metabolites from biofilms of Candida albicans fungi and Staphylococcus aureus bacteria. These microorganisms are very frequent in users of dentures: prior research found both in 30%-40% of subjects examined.
Oral microbiota is known to play an important role in the development of cancer. Genetic markers associated with the presence of microorganisms have been identified for some types, such as stomach cancer, but there is no consensus regarding the most prevalent genes linked to head and neck cancer, and no molecular markers had hitherto been found for this disease, especially HPV-negative cancer, which has a worse prognosis.
According to a report on the study published in Frontiers in Cellular and Infection Microbiology, metabolites from C. albicans and S. aureus biofilms can endanger the homeostasis of normal and neoplastic oral epithelial cells, altering the expression of important genes such as CDKN1A, Bcl-2, PI3K, BRAF, hRAS and mTOR, impairing cell viability and survival, and disrupting the cell cycle profile.
The study was supported by FAPESP and the National Council for Scientific and Technological Development (CNPq). The project was also awarded funding by Colombia’s COLCIENCIAS and SAPIENCIA agencies (2015 call for doctorates abroad), and involved partnerships with UNESP’s Araraquara Dental School (FOAr) and Araraquara School of Pharmaceutical Sciences (FCFAr).
Understanding the cell cycle is important because cancer entails unchecked cell division and growth, with tumor cells potentially invading tissues and organs throughout the body. Failure of the inhibitors of this cycle and excessive signaling by cell division regulators can lead to tumor progression.
The oral microbiome is a diversified community of microorganisms with as many as 700 species of viruses, protozoans, bacteria and fungi. When biofilms develop, they produce metabolites that alter the immune response and can lead to chronic inflammation and even production of cancerous substances.
According to Paula Aboud Barbugli, a professor at FOAr-UNESP and co-leader of the study, the findings show that “molecules secreted by these microorganisms in biofilms may modulate host cell activities even far away from the primary infection site”.
For Carlos Eduardo Vergani, also a professor at FOAr-UNESP and principal investigator for the project, the results serve as a warning on the treatment of cancer patients who have dentures. “Control of biofilms, including denture and oral cavity hygiene, is extremely important to minimize inflammatory processes, as shown by our prior research and the study just published, which points to interference with the expression of genes associated with tumor progression,” Vergani told Agência FAPESP.
Another study led by Vergani and published in 2017 showed that soluble factors in methicillin-sensitive C. albicans and S. aureus biofilm promoted cell death and inflammatory responses.
According to a report issued in March by Brazil’s National Cancer Institute (INCA), some 22,800 new cases of laryngeal and oral cavity cancer are reported each year, most of them in male patients.
Future
The COVID-19 pandemic forced the group to interrupt the clinical trial they had planned to conduct on the basis of their research. Barbugli said a PhD project supervised by another member of the group and approved by the Ministry of Health’s Research Ethics Committee (CONEP) will investigate the prevalence of C. albicans and S. aureus biofilms in the dentures and oral cavities of head and neck cancer patients treated at Araraquara’s Santa Casa de Misericórdia Hospital to understand how they influence prognosis in these cases.
For Vergani, the results obtained so far pave the way for future metabolomics and proteomics research into oral biofilms.
###
About São Paulo Research Foundation (FAPESP)
The São Paulo Research Foundation (FAPESP) is a public institution with the mission of supporting scientific research in all fields of knowledge by awarding scholarships, fellowships and grants to investigators linked with higher education and research institutions in the State of São Paulo, Brazil. FAPESP is aware that the very best research can only be done by working with the best researchers internationally. Therefore, it has established partnerships with funding agencies, higher education, private companies, and research organizations in other countries known for the quality of their research and has been encouraging scientists funded by its grants to further develop their international collaboration. You can learn more about FAPESP at http://www.
Media Contact
heloisa reinert
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




