The research adds to a body of work devoted to understanding the protein’s role in cell biology and health
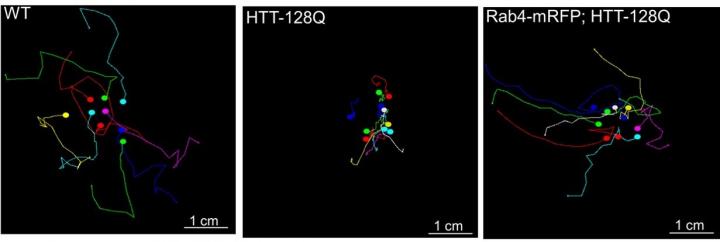
Credit: Credit: White, J.A., Krzystek, T.J., Hoffmar-Glennon, H. et al., Acta Neuropathologica Communciations, July 2020. The image is adapted from a graphic in the journal article, which is distributed under Creative…
BUFFALO, N.Y. — A new study reveals intricate details about the biology of the huntingtin protein (HTT), which is responsible for Huntington’s disease.
The research focuses on axonal transport — the way in which vital materials travel along pathways called axons inside nerve cells, or neurons.
Scientists found that HTT sometimes journeys along these roadways in cellular vehicles (called vesicles) that also carry freight including a protein called Rab4. Other materials that may be present in these shipments include proteins essential to the health and function of nerve cells, scientists conclude.
The findings also point to a potential treatment avenue: In fruit fly larvae, a mutated form of HTT that causes Huntington’s disease disrupted the normal movement of vesicles holding HTT and Rab4. This led to problems such as defects in a part of the nerve cell called the synapse; difficulties with crawling; and decreased lifespan, the study found. But when larvae carrying the HTT mutation were engineered to also produce excess Rab4, some of these symptoms — including abnormalities in synapses, crawling and lifespan — were less pronounced.
“When it comes to finding therapies for neurological diseases, a lot of the research focuses on the pathology and aggregation of proteins, but that may not be the actual cause,” says Shermali Gunawardena, PhD, associate professor of biological sciences in the University at Buffalo College of Arts and Sciences. “We want to try to figure out what might be going on earlier. We want to come in from a different angle and say, ‘OK, are huntingtin and Rab4 normally present together in healthy neurons? And then, what are they doing in the context of a disease state?’ “
“This research helps shed light on the normal function of huntingtin and how it is altered in disease,” says co-first author Joseph A. White II, a UB biological sciences PhD graduate who is now a postdoctoral researcher at Duke University. “Based on what we’re seeing, HTT seems to be important for the transport of a particular type of vesicle known as endosomes within neurons. We believe this presents a potential avenue for therapeutics aimed at improving endosomal transport in Huntington’s disease patients.”
Gunawardena led the study with first authors White and Thomas J. Krzystek, a UB PhD student in biological sciences.
The research, published on July 1 in Acta Neuropathologica Communications, builds on past work in Gunawardena’s lab. Previously, her team showed that mutated HTT can also perturb the movement of some other Rab proteins. The findings suggest that potential treatments focusing on axonal pathways may also need to be multifaceted, involving a number of drugs targeting different problems, Gunawardena says.
“The idea behind our work is to tease out these details about what’s going on inside neurons,” she says. “It’s really difficult to isolate what’s going on inside the organism. But from what we have done in the lab, we can say now that huntingtin is in these different, specific kinds of cargoes. I think the bottom line is that this protein is doing a lot of different things.”
“Model organisms, like the fruit fly, are excellent tools we can use to quickly investigate a variety of proteins and pathways related to human diseases,” Krzystek says. “Our study highlights the advantage of using these simpler organisms to discover new information that we can then test directly in more complex systems. Through this, we learned about what huntingtin might be doing with Rab4 in healthy neurons, and how this role may become disrupted in Huntington’s disease.”
###
The study was funded by the National Institute of Neurological Disorders and Stroke, part of the National Institutes of Health. Support for individual authors came from the Mark Diamond Research Fund, the Beverly Petterson Bishop and Charles W. Bishop Neuroscience Travel Award, the Center for Undergraduate Research and Creative Activities, the Philip G. Miles Undergraduate Summer Research Fund, and the Darryl Raszl Undergraduate Research Fellowship, all at UB.
Co-authors of the research included other scientists from UB and Cornell University.
Media Contact
Charlotte Hsu
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.




