Credit: Authors
CO2 electroreduction reaction driven by renewable electricity is an effective way to reduce the concentration of CO2 in the atmosphere and alleviate environmental problems such as global warming. It can convert CO2 into valuable products (such as CO, HCOOH, CH4) to realize effective carbon cycle. At present, the reported highly efficient electrocatalysts for electrocatalytic CO2 reduction reaction (CO2RR) are mainly concentrated on nanomaterials. Among them, N-doped or N-heterocyclic nanostructured electrocatalysts have made important progress in reduction product conversion and Faraday efficiency. However, due to the lack of accurate and clear structural information and other influencing factors (including defects and impurities), it is still difficult to determine the activity of N sites in these electrocatalysts.
In this case, the crystal electrocatalysts with clear crystal structure have great advantages in solving the above problems, because their accurate structure information can provide a visual research platform for identifying catalytic active sites and studying reaction mechanism. Metalloporphyrin complexes applied in CO2RR have many advantages. Among them, the rigid ring with conjugated π – electron system of metalloporphyrin is favorable to the rapid electron migration. More importantly, their clear molecular structure information and structural tunability are very helpful for studying reaction mechanisms and rationally optimizing catalytic performance.
Based on this, establishing a reasonable crystal model system to accurately identify the activity of catalytic sites in electrocatalysis is very important for the development of electrocatalytic CO2RR.
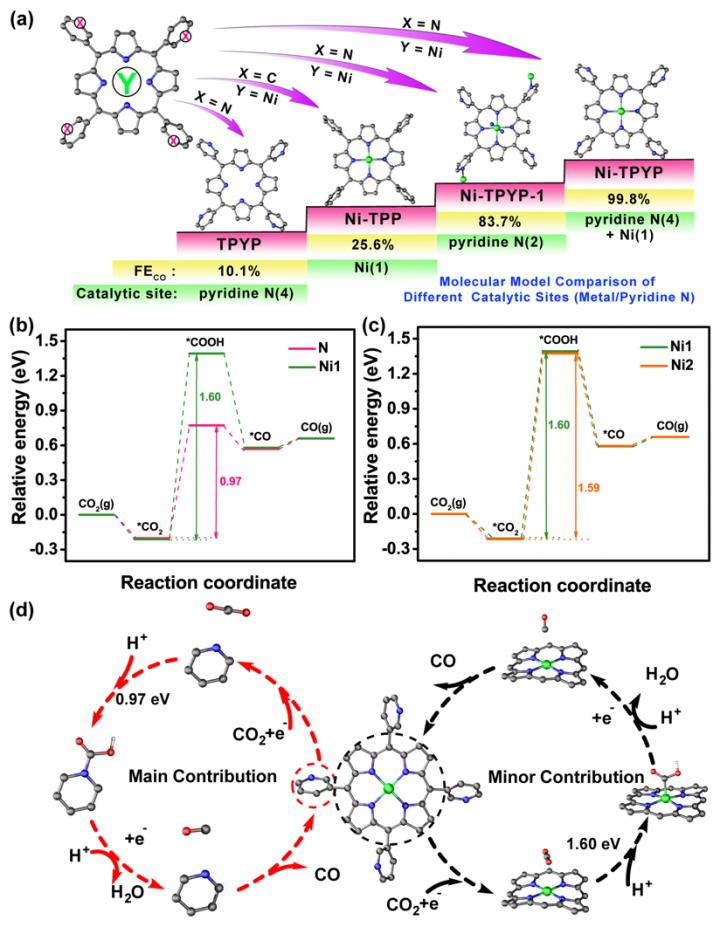
In a new research paper published in National Science Review (NSR), the research group of professor Ya-Qian Lan of Nanjing Normal University, for the first time, established a crystal supramolecular coordination compound model system (including Ni-TPYP, Ni-TPYP-1 and Ni-TPP, as shown in Figure 1) to identify structurally the catalytic activity of pyridine N for electrocatalytic CO2RR. This work is of great significance for understanding the catalytic activity and reaction mechanism of N-doped or N-heterocyclic nanostructured electrocatalysts in electrocatalytic CO2RR.
Experimental and theoretical calculations show that the rate determining step (RDS) of electrocatalytic CO2RR in this system is the formation of *COOH. In this step, the energy required for Ni active site (denoted as Ni1) in Ni-TPYP and Ni active site (denoted as Ni2) in Ni-TPP are almost the same (1.60 eV and 1.59 eV) and both are higher than that of active pyridine N (denoted as N, 0.97 eV) in Ni-TPYP, indicating that N site has higher CO2 electroreduction activity than Ni2 and Ni1 sites, that is, active pyridine N is a more suitable catalytic active site.
###
See the article:
Sheng-Nan Sun,† Ning Li,† Jiang Liu,* Wen-Xin Ji, Long-Zhang Dong, Yi-Rong Wang, and Ya-Qian Lan*
Identification of the Activity Source of CO2 Electroreduction by Strategic Catalytic Site Distribution in Stable Supramolecular Structure System
https:/
Media Contact
Ya-Qian Lan
[email protected]
Related Journal Article
http://dx.




