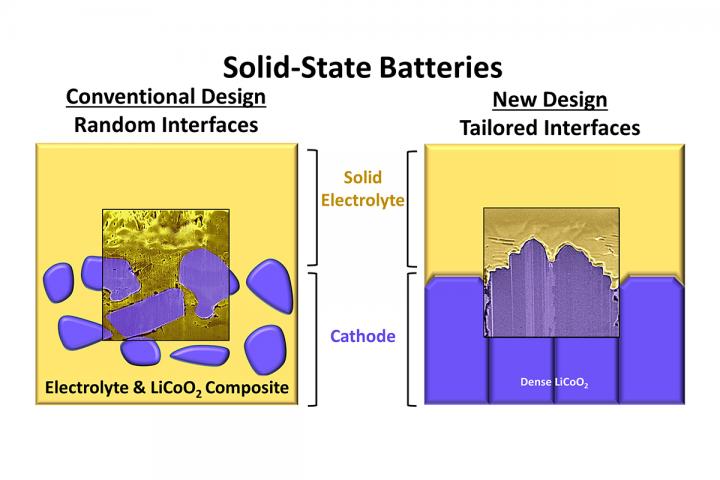
Credit: Graphic courtesy Beniamin Zahiri and Paul Braun
CHAMPAIGN, Ill. — Solid-state batteries pack a lot of energy into a small space, but their electrodes are not good at keeping in touch with their electrolytes. Liquid electrolytes reach every nook and cranny of an electrode to spark energy, but liquids take up space without storing energy and fail over time. Researchers are now putting solid electrolytes in touch with electrodes made of strategically arranged materials – at the atomic level – and the results are helping drive better solid-state battery technologies.
A new study, led by University of Illinois Urbana-Champaign materials science and engineering professor Paul Braun, postdoctoral research associate Beniamin Zahiri, and Xerion Advanced Battery Corp. director of research and development John Cook, demonstrates how control over the atomic alignment of solid materials can improve the cathode-solid electrolyte interface and stability in solid-state batteries. The results are published in the journal Nature Materials.
“With batteries, it’s not just materials that are important, but also how the atoms on the surfaces of those materials are arranged,” Zahiri said. “Currently, solid-state battery electrodes contain materials with a large diversity of surface atom arrangements. This leads to a seemingly infinite number of electrode-solid electrolyte contact interface possibilities, all with different levels of chemical reactivity. We are interested in finding which arrangements lead to practical improvements in battery cycle life, energy density and power.”
The researchers said an electrolyte’s stability controls how many charging and discharging cycles a battery can handle before it starts to lose power. Because of this, scientists are in a race to find the most stable electrolyte materials.
“In the rush to find stable solid electrolyte materials, developers have sort of lost sight of the importance of what is happening in that very thin interface between electrolyte and electrode,” Zahiri said. “But the stability of the electrolyte will not matter if the connection between it and the electrodes cannot be evaluated in an efficient way.”
In the lab, the team built electrodes containing sodium and lithium ions with specific atomic arrangements. They found correlations between battery performance and interface atomic arrangement in both the lithium- and sodium-based solid-state batteries. They also discovered that minimizing the interface surface area and controlling the electrodes’ atomic alignment is key to both understanding the nature of interface instabilities and improving cell performance.
“This is a new paradigm for how to evaluate all the important solid electrolytes available today,” Cook said. “Before this, we were largely just guessing what electrode-solid electrolyte interface structures gave the best performance, but now we can test this and find the best combination of materials and atomic orientations.”
As demonstrated by co-author mechanical science and engineering professor Elif Ertekin and her group, having this level of control gave the researchers the information needed to run atomic simulations that they hypothesize will lead to even better electrolyte materials in the future, the researchers said.
“We think this will teach us a lot about how to investigate emerging solid electronics,” Braun said. “We are not trying to invent new solid electrolytes; the materials world is doing a great job with that already. Our methodology will allow others to precisely measure the interfacial properties of their new materials, something that has otherwise been very difficult to determine.”
###
Braun is the Materials Research Laboratory director and an affiliate of mechanical science and engineering, chemistry, the Beckman Institute of Advanced Science and Technology and the Holonyak Micro and Nanotechnology Laboratory at Illinois. Ertekin is the director of mechanics programs in mechanical sciences and engineering and also is affiliated with the Materials Research Laboratory and National Center for Supercomputing Applications.
The Department of Defense, the United States Army and the Army Corps of Engineers supported this study.
Editor’s notes:
To reach Paul Braun, call 217-244-7293; email [email protected].
The paper “Revealing the role of the cathode-electrolyte interface on solid-state batteries'” is available online and from the U. of I. News Bureau. DOI: 10.1038/s41563-021-01016-0.
Media Contact
Lois Yoksoulian
[email protected]
Related Journal Article
http://dx.




