Investigator-driven networks enter COVID-19 research space
Credit: HVTN design: Lisa Donohue
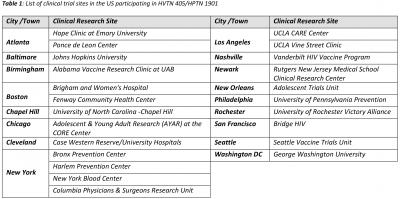
SEATTLE, MAY 14, 2020 – The NIH-funded HIV Vaccine Trials Network (HVTN) and HIV Prevention Trials Network (HPTN) have initiated their first clinical trial in response to COVID-19. The study, called HVTN 405/HPTN 1901, is underway at clinical trial sites across North and South America and will describe immune responses in study participants with a history of infection with SARS-CoV-2, the virus that causes COVID-19.
Since COVID-19 first emerged in late 2019 in China, more than 4.3 million cases have been reported, and the disease has led to more than 294,000 deaths globally1. Recent updates by the US Centers for Disease Control and Prevention confirmed over 1.3 million cases of COVID-19 as of May 2020 in the US. Over 80,000 deaths have been reported in the US alone2. Since before COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020, countries have been working on measures to save lives and prevent the transmission of the virus.
“The study includes an important milestone needed for SARS-CoV-2 vaccine development and will help create the infrastructure required to do sophisticated vaccine trials,” said Dr. Larry Corey M.D., Protocol Chair and Principal Investigator, HVTN Leadership Operations Center which is based at the Fred Hutchinson Cancer Research Center.
HVTN 405/HPTN 1901 aims to enroll approximately 400 study participants aged 18 and older who tested positive for SARS-CoV-2 and have since recovered. Participants will have one required clinic visit and will have the option to participate in additional clinic visits two, four and twelve months after the initial visit. Each visit includes a blood draw and optional nasal sampling procedures. Individuals who still have symptoms of infection or asymptomatic individuals less than two weeks from the date of their last positive test will not be enrolled.
“Our collaborative NIH-funded networks are fully committed to using our well-established research infrastructures to pivot into the prevention of COVID-19,” said Myron Cohen, M.D., HPTN co-principal investigator, and director of the Institute for Global Health and Infectious Diseases at the University of North Carolina-Chapel Hill School of Medicine. “This study is one of many that will help us better understand how the human immune system responds to SARS-CoV-2.”
SARS-CoV-2 is the most infectious of three coronaviruses that have caused recent epidemics resulting in significant morbidity and mortality in humans in the past 20 years. Given the extent to which the disease has spread, mathematical modeling suggests that SARS-CoV-2 is likely to become endemic in human populations3. A main aim of HVTN 405/HPTN 1901 is to develop antibody assays that can reliably detect SARS-CoV-2 infection in vaccine and monoclonal antibody studies to prevent COVID-19. Detailed tests of different antibodies collected from blood and nasal samples will be assessed for their diagnostic utility and the antibodies will be analyzed for their potential to provide immune protection.
Prevention tools like a vaccine are critical. This study will describe the body’s natural immune responses to SARS-CoV-2, thus illuminating the path to the development and testing of a safe and effective vaccine. Vaccines are not only essential to prevent new infections and reduce morbidity and mortality, but they will also aid in a return to a thriving social and economic global infrastructure.
“The HVTN and HPTN have been recognized for our history of successfully engaging communities in the HIV prevention studies we have been doing for 20 years,” said Gail Broder, MHS, Community Engagement Lead for HVTN 405/HPTN 1901 and HVTN Senior Community Engagement Project Manager. “It’s a privilege to turn that experience and expertise to the COVID-19 fight, where we will need to connect with new populations and new stakeholders that are impacted by this pandemic. We know that communities will continue to be the bedrock of our success in implementing this clinical trial,” Broder concluded.
Since its inception in 1999, the HVTN has conducted more than 80 clinical trials involving more than 22,000 study participants globally. The Network has forged global public-private partnerships, and through in-country and strong community relationships, continues to contribute to the global response to help end HIV. Using its infrastructure of global clinical trial sites, laboratories, and biostatistical expertise, the HVTN and partners hope to make significant contributions to the global COVID-19 response.
###
HVTN 405/HPTN 1901 is sponsored by NIH’s National Institute of Allergy and Infectious Diseases. Interested individuals can email [email protected] for more information.
References:
1. Johns Hopkins University & Medicine – https:/
2. US Centers for Disease Control and Prevention – https:/
3. Yang CY, Wang J. A mathematical model for the novel coronavirus epidemic in Wuhan, China. Math Biosci Eng. 2020;17(3):2708-24.
About Fred Hutch:
At Fred Hutchinson Cancer Research Center, home to three Nobel laureates, interdisciplinary teams of world-renowned scientists seek new and innovative ways to prevent, diagnose and treat cancer, HIV/AIDS and other life-threatening diseases. Fred Hutch’s pioneering work in bone marrow transplantation led to the development of immunotherapy, which harnesses the power of the immune system to treat cancer. An independent, nonprofit research institute based in Seattle, Fred Hutch houses the nation’s first National Cancer Institute-funded cancer prevention research program, as well as the clinical coordinating center of the Women’s Health Initiative and the international headquarters of the NIAID-funded HIV Vaccine Trials Network.
About the HPTN:
The HIV Prevention Trials Network (HPTN) is a worldwide collaborative clinical trials network that brings together investigators, ethicists, community members, and other partners to develop and test the safety and efficacy of interventions designed to prevent the acquisition and transmission of HIV. The U.S. National Institute of Allergy and Infectious Diseases, the U.S. National Institute of Mental Health, and the U.S. National Institute on Drug Abuse, all components of the U.S. National Institutes of Health, co-fund the HPTN. The HPTN has collaborated with more than 85 clinical research sites in 19 countries to evaluate new HIV prevention interventions and strategies in populations that bear a disproportionate burden of infection. The HPTN research agenda – more than 50 trials ongoing or completed with over 161,000 participants enrolled and evaluated – is focused primarily on the use of integrated strategies: use of antiretroviral drugs (antiretroviral therapy and pre-exposure prophylaxis); interventions for substance abuse, particularly injection drug use; behavioral risk reduction interventions and structural interventions. For more information, visit hptn.org.
Media Contact
Aziel Gangerdine
[email protected]
Original Source
http://www.




