Credit: JAIST
Researchers at Japan Advanced Institute of Science and Technology (JAIST) and Dalian Institute of Chemical Physics, Chinese Academy of Sciences have successfully established a universal synthetic design using porous organic polymers (POPs) for fuel cell electrolyte, according to an Editor’s choice hot article published in the journal Materials Chemistry Frontiers.
Development of new materials for cost-effective technologies is urgent and necessary to bring about an environmentally sustainable society. Polymer electrolyte fuel cells have high expectations for a clean energy system that can support environmental protection. They must be able to split a molecule of hydrogen into positively charged protons and negatively charged electrons. For this purpose, polymeric materials with high proton conductivity are needed. Electrons do not pass through the material, only protons pass through, so we can be extracted as electricity.
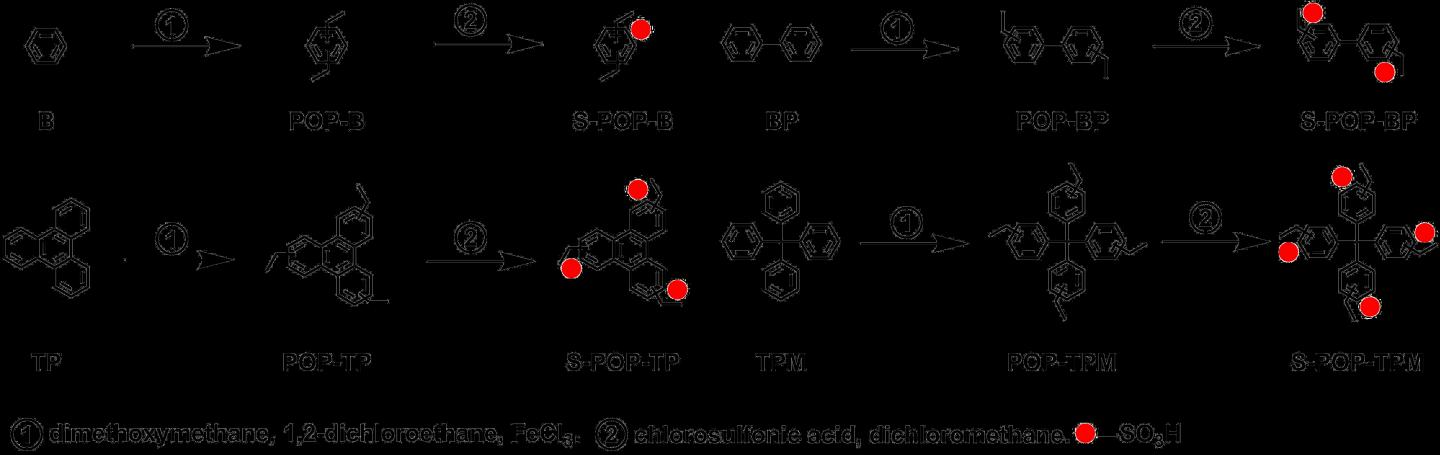
Research has shown simple, universal, and cost-effective synthetic strategy for gaining highly proton-conductive POPs as shown in Scheme 1. They show excellent proton conductivity of 10-2 to 10-1 S cm-1.
In the research so far, there were problems that the synthetic method using POPs was complicated and the skeleton was limited. In order to establish the synthetic strategy universal for practical applications, we were able to try various skeletons as POPs and established the synthetic method applicable to almost all aromatic-based materials, says materials scientist Yuki Nagao of JAIST, who has been researching proton-conducting materials for many years.
They divided the synthetic steps into two steps. First, a porous organic polymer was synthesized. Second, a post-sulfonation strategy was adopted which then introduced sulfonic acid groups through the pores. The catalyst used during synthesis causes deterioration of the material during fuel cell operation, but it could also be removed by using the pores. A remarkable conductivity of S-POP-TPM (Scheme 1) was recorded on 2.7 × 10-2 and 1.0 × 10-1 S cm-1 under 25 and 80 °C at 95% RH, respectively.
“Results of this study indicate that the structure of sulfonated POPs offers a simple and universal means for evolving structural design for highly proton-conductive materials.,” explains Zhongping Li, who is the first author of this work. A step forward towards a hydrogen society.
###
Media Contact
Yuki Nagao
[email protected]
Related Journal Article
http://dx.