Study finds biological process that helps underdeveloped lungs fight infections
Credit: Cincinnati Children’s
CINCINNATI – The continuing epidemic of pre-term births includes this stark reality: tiny, fragile babies are born with underdeveloped lungs and prone to lifelong respiratory infections and related chronic illnesses.
Cincinnati Children’s researchers report in Immunology the discovery of a complex biological processes that in premature lungs stimulates production of Type 3 innate lymphoid cells in air sacs called alveolar. Born without these cells, premature infants’ lungs cannot mount an effective immune defense against microbial lung infections. This opens the door to bacterial pneumonia and other severe respiratory illnesses, according study authors at the Cincinnati Children’s Perinatal Institute.
“This study gives us important new information that helps us develop new and cost effective methods to boost innate lung immunity in preterm babies. This could help them develop lifelong pulmonary resistance to respiratory infections,” said Hitesh Deshmukh, MD, PhD, senior study author and a Cincinnati Children’s neonatologist.
Deshmukh and his colleagues now plan pre-clinical studies in pre-term laboratory mouse pups to find out if they can harness their newly discovered biogenetic processes to stimulate production of Type 3 innate lymphoid cells in developing lungs. Their theory is that this will promote durable immune defenses in the mouse lungs against infection and tissue damaging inflammation.
A Biological Ballet
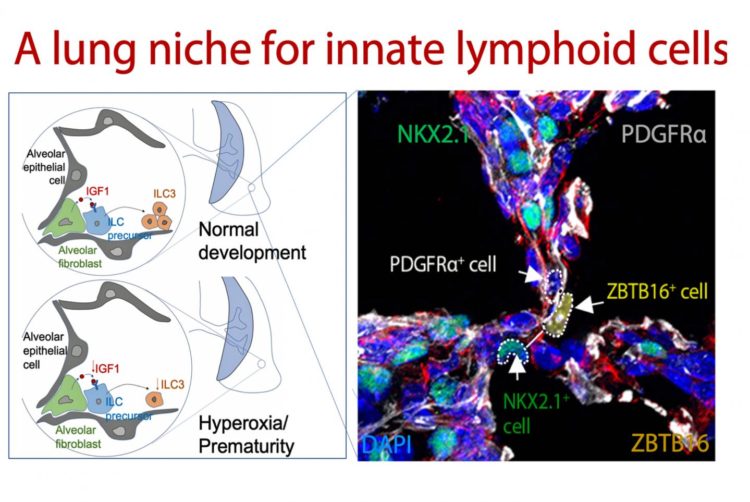
The current study reveals a complex interplay between beneficial bacteria in the intestines, the maturation of lung tissues and the growth of immune cells in what the researchers call the gut-lung axis. It happens in neonates during an early and critical development window of the newborn lung, according to their report.
Commensal bacteria from the gut stimulate the production of Type 3 innate lymphoid cells. This occurs in early alveolar cells called fibroblasts when biological cues from the gut stimulate production of a hormone called insulin-like growth factor 1 (IGF1). IGF1 orchestrates expansion and maturation of early pulmonary innate lymphoid cells, the study shows.
When researchers deleted pulmonary IGF1 in the lungs of baby mice, it interrupted the biogenic development of Type 3 innate lymphoid cells and made the mice susceptible to lung infections and pneumonia. They confirmed this by analyzing the underdeveloped lungs of premature mouse pups and donated lung tissues from the families of premature human infants.
The findings have potential clinical implications because premature infants are depleted of helpful gut bacteria by the need for oxygen ventilation to help them breath. This is exacerbated by what Deshmukh called a “double whammy” when the babies have to be administered antibiotics to fight their vulnerability to infection.
The preclinical study on mice to test potential therapies will include administering helpful gut bacteria and pulmonary IGF1 to see if this stimulates to development of a robust immune defense. Deshmukh said therapeutic administration of commensal bacteria and the IGF1 hormone are both being tested clinical in humans for other medical conditions.
Never Ending Challenge
Although medical science and technology continue to advance, premature birth and its complications remain a major challenge as the largest contributors to global infant death, according to the March of Dimes. In 2018, the most recent year statistically available, the preterm birth rate in the United States worsened for the fourth straight year, from 9.63 percent in 2015 to 10.02 percent in 2018.
Bacterial pneumonia kills more than one million infants around the world each year, study authors note.
Prior to the new study in Science Immunity the researchers published previous papers in Nature Medicine and Science Translational Medicine establishing the critical of role of the Gut-Lung Axis molecular interplay and the potentially harmful impact of aggressive antibiotic regimens in premature infants.
###
Funding support came in part from the National Institutes of Health (K08HD084686, R01HL142708, R01DK114213, DK116868, R01HL131634, R01HL122300 K08HL140178 and U01HL122642), the Francis Family Foundation, and a Pew Charitable Trust Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Study DOI is: 10.1016/j.immuni.2020.01.005
Media Contact
Nick Miller
[email protected]
513-803-6035
Related Journal Article
http://dx.