In a breakthrough with applicability across many flu strains, researchers find a potential small molecule that mimics antibodies to treat disease
Credit: Wilson lab
LA JOLLA, CA–March 8, 2019–A team of researchers from Scripps Research and Janssen Research & Development LLC has discovered an orally active small molecule that neutralizes influenza A group 1 viruses, the most common flu strains. Scientists uncovered the potential therapy in a large chemical library and optimized lead compounds to produce JNJ-4796. Now they have proven its effectiveness against influenza in mice.
“What’s exciting is that this molecule has the potential to target different strains and subtypes of influenza virus,” says co-senior investigator Ian Wilson, PhD, Hansen Professor of Structural Biology at Scripps Research. Not only do influenza A type 1 viruses account for many of the seasonal flu strains, he says, but also pandemic strains, such as H1N1 and H5N1.
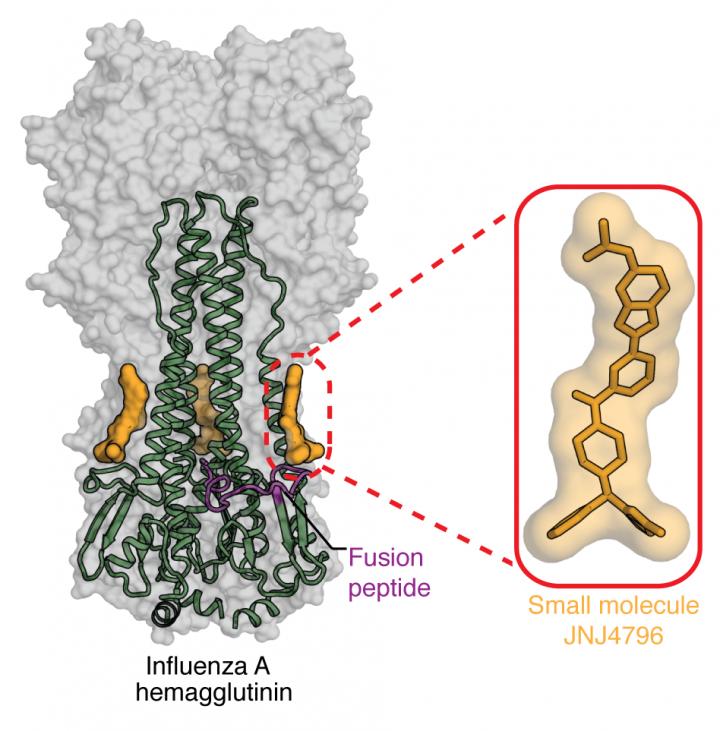
In a typical year, influenza sickens 5 to 20 percent of the U.S. population, leading to more than 200,000 hospitalizations and 36,000 deaths, according to the Centers for Disease Control and Prevention. Strains of influenza A virus are categorized into group 1 and group 2, and then into further subtypes. Antibodies against the virus often only recognize a handful of similar strains. However, a decade ago, Wilson was part of a team of researchers who described a “super- antibody” called CR6261–isolated from a healthy human–that recognized a part of the virus called the hemagglutinin (HA) stem, which is present across influenza strains.
Since that time, researchers have been striving to use these super-antibodies, known as broadly neutralizing antibodies (bnAbs), to develop approaches which could lead to universal flu vaccines and treatments. However, antibodies themselves are large molecules that can only be given through injections, never as an oral drug, which makes them impractical for treating patients at home. A more practical drug, Wilson theorized, would consist of a small molecule that recognizes the HA stem in the same way as the antibody.
In 2017, collaborators designed an artificial peptide that bound to the same region of the HA stem as CR6261 using information on how other stem bnAbs, FI6v3 and CR9114, bind to and neutralize influenza virus. In the new work, published online in the journal Science, they’ve taken a different approach to mimicking the antibody’s actions.
The researchers turned to a library of more than 500,000 drug-like molecules and, in a high-throughput experiment, tested whether any of the compounds could displace a small protein HB80.4 mimic of bnAb CR6261 from the influenza type 1 HA stem, suggesting that such molecules attached to the same spot on the virus. Multiple molecules turned up as positive on the screen, and optimization to produce better binding and pharmacokinetic properties resulted in JNJ-4796 being the most promising inhibitor. Structural work in the Wilson lab revealed the close mimicry of how JNJ4796 bound to the HA stem compared to bnAb CR6261.
The team went on to show that when mice were given JNJ-4796 for seven days, beginning one day before being infected with 25 times the normally-lethal dose of H1N1 influenza, 100 percent of the mice survived. Moreover, when JNJ-4796 was incubated with human bronchial epithelial cells–airway cells affected by a flu infection–the compound dramatically lowered levels of the virus present in the tissue 96 hours after infection.
While the current version of JNJ-4796 targets the HA stem of influenza A group 1 viruses, the study revealed structural information on how the molecule binds to the HA stem and what changes could possibly make it act more broadly against other influenza strains. While this manuscript suggests that this may be a potential strategy for the treatment of influenza A, this study was pre-clinical, and further studies would be warranted to pursue this approach.
In addition to pointing towards a potential strategy for the design of new drugs to treat influenza, the new work opens the door to designing other antiviral drugs using a similar antibody-inspired approach.
“The molecule is proof-of-principle that antibody-guided drug discovery can work,” says co-first author Rameshwar U. Kadam, PhD, a senior research associate at Scripps Research. “We can now apply this approach to a whole host of other viruses.”
Kadam adds that HIV and Ebola–for which neutralizing antibodies are known–would be especially useful to study in the same way. While antibodies can cost thousands of dollars per dose, small molecules are cheaper, more shelf-stable, and can be developed into oral compounds that are more convenient for patients. Further research would be required.
###
In addition to Wilson, van Dongen, and Kadam, authors of the study, “A small-molecule fusion inhibitor of influenza virus is orally active in mice,” include Divita Garg and Wenli Yu of Scripps Research; and Jarek Juraszek, Edward Lawson, Boerries Brandenburg, Frederike Schmitz, Wim B. G. Schepens, Bart Stoops, Harry A. van Diepen, Mandy Jongeneelen, Chan Tang, Jan Vermond, Alida van Eijgen-Obregoso Real, Sven Blokland, Wouter Goutier, Ellen Lanckacker, Jaco M. Klap, Daniëlle C. G. Peeters, Jin Wu, Christophe Buyck, Tim H. M. Jonckers, Dirk Roymans, Peter Roevens, Ronald Vogels, Wouter Koudstaal, Robert H. E. Friesen, Pierre Raboisson, Dashyant Dhanak, and Jaap Goudsmit of Janssen.
This work was supported by funding from the National Institutes of Health (R56 AI117675 and R56 AI127371) and the Swiss National Science Foundation. Use of the Stanford Synchrotron Radiation Lightsource to collect x-ray datasets was supported by the U.S. Department of Energy (contract DE-AC02-76SF00515). The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institute of General Medical Sciences (including P41GM103393).
A patent application related to this work has been filed by some of the authors.
About Scripps Research
Scripps Research ranks as the most influential scientific organization in the world, unparalleled in propelling innovation in science and medicine. Our unique structure merges foundational studies in biology, chemistry and computer science with translational science to produce the next generation of drugs and advances in digital and precision medicine. Scientists in the institute’s five academic research departments work hand-in-hand with researchers of the Scripps Research Translational Institute and Calibr. Together, we cultivate the next generation of scientific leaders and expand the frontiers of knowledge to drive innovation that improves lives around the planet.
Media Contact
Chris Emery
[email protected]
Related Journal Article
http://dx.




