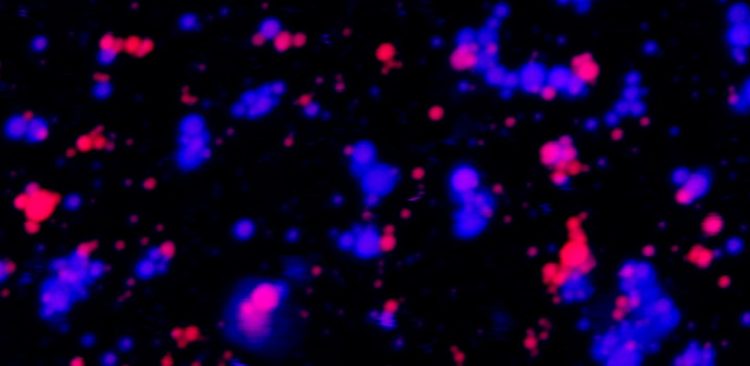
Physicists use model systems to explore the forces that may be at play
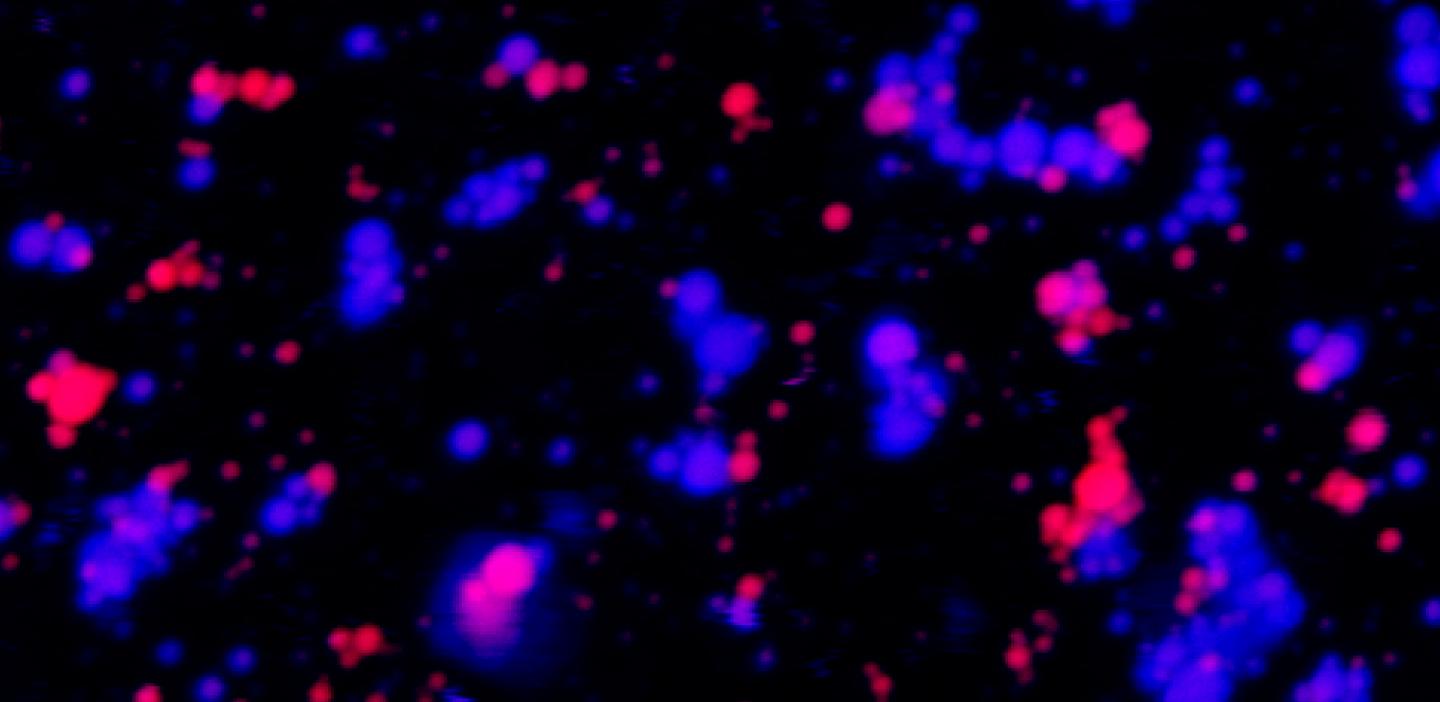
Credit: Credit: Ibraheem Alshareedah / Priya Banerjee Laboratory
BUFFALO, N.Y. — New research may help to explain an intriguing phenomenon inside human cells: how wall-less liquid organelles are able to coexist as separate entities instead of just merging together.
These structures, called membrane-less organelles (MLOs), are liquid droplets made from proteins and RNA, with each droplet holding both materials. The organelles play a crucial role in organizing the internal contents of cells, and can serve as a center of biochemical activity, recruiting molecules needed to carry out essential cellular reactions.
But how different droplets stay apart from each other remains a mystery. Why don’t they always just combine to form bigger droplets?
“These organelles don’t have any membrane, and hence, common intuition would tell you that they are free to mix,” says Priya Banerjee, PhD, assistant professor of physics in the University at Buffalo College of Arts and Sciences.
Banerjee is the lead researcher on the new study, which explores why this doesn’t happen.
Co-authors of the research include first author and physics PhD student Ibraheem Alshareedah; physics PhD student Taranpreet Kaur; undergraduate Jason Ngo; physics and math undergraduate Hannah Seppala; biomedical engineering undergraduate Liz-Audrey Djomnang Kounatse; and physics postdoctoral researchers Wei Wang and Mahdi Moosa. All are from UB.
Droplets won’t mix easily if they take on a gel-like state
The results — published on Aug. 22 in the Journal of the American Chemical Society — point to the chemical structure of protein and RNA molecules within the droplets as one key factor that may prevent MLOs from mixing.
The team found that certain types of RNA and proteins are “stickier” than others, enabling them to form gelatinous droplets that don’t fuse easily with other droplets in the same viscoelastic state. Specifically, droplets are more likely to be gel-like when they contain RNA molecules rich in a building block called purine, and proteins rich in an amino acid called arginine.
The experiments did not take place in cells. Instead, the findings were based on tests done on model systems consisting of RNA and a droplet-forming protein called fused in sarcoma (FUS) floating in a buffer solution.
One reason FUS is of interest to researchers is its potential connection to the neurodegenerative disease amyotrophic lateral sclerosis (ALS). As Banerjee explains, arginine-rich protein molecules are associated with a prevalent form of the disease, known as c9orf72-mediated ALS.
“Our finding points to a special role of arginine-rich proteins in determining the material state — liquid vs. gel — of membrane-less organelles,” Banerjee says. “This study may be important in understanding how ALS-linked arginine-rich proteins may alter the viscoelastic state of RNA-rich MLOs.”
In addition to providing insight into why MLOs resist mixing (due to enhanced viscoelasticity), the study probed the role of RNA in the formation and dissolution of liquid organelles containing FUS. The research found that for the type of droplet being studied, adding low concentrations of RNA to a solution containing the proteins caused droplets to form. But as more RNA was added, the droplets then dissolved.
“There’s usually a very small window where these droplets exist, but the window is significantly wider for arginine-rich proteins,” Banerjee says.
The complicated life of liquid organelles
The new paper is the latest in a series of studies that Banerjee’s group has conducted to explore forces governing the creation, maintenance and dissolution of MLOs.
Though the team uses model systems to examine individual properties of the droplets, it’s likely that many forces work together in a cell to determine the behavior and function of the organelles, he says. There may be multiple other mechanisms, for example, that cause MLOs to take on a gelatinous state or otherwise refuse to mix.
“Cells are enormously complex, with many different molecules undergoing different processes that come together at the same time to influence what goes on inside MLOs,” Banerjee says. “By using model systems, we are able to better understand how one particular variable may impact the formation and dissolution of these organelles. And we do expect to see these same forces at play in nature, inside cells.”
###
Banerjee recently received a grant from the National Institute on Aging, part of the National Institutes of Health, to further study MLOs. The project is expected to expand the understanding on how MLOs change over time.
Media Contact
Charlotte Hsu
[email protected]
716-645-4655
Original Source
http://www.
Related Journal Article
http://dx.