Credit: Ojeda DS et al., 2021, PLOS Pathogens, CC-BY 4.0
Researchers have developed and applied a robust, versatile antibody test to assist health authorities in managing the coronavirus disease 2019 (COVID-19) pandemic, according to a study published January 14 in the open-access journal PLOS Pathogens by Andrea Gamarnik of the Fundación Instituto Leloir-CONICET in Argentina, and colleagues.
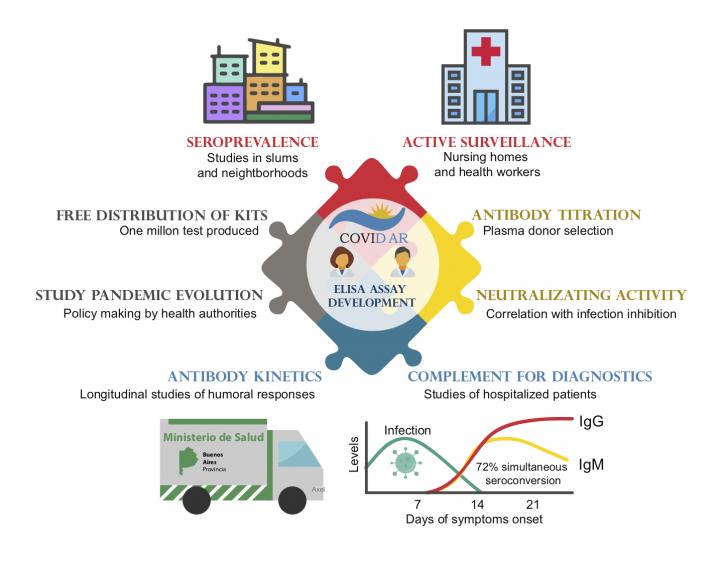
As of November 22, there have been more than 57 million COVID-19 cases worldwide since the start of the pandemic, resulting in more than one million deaths globally. Surveillance and testing play an important role in controlling viral spread. In the new study, Gamarnik and colleagues developed and applied a robust, versatile test to detect antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the virus that causes COVID-19. Specifically, the enzyme-linked immunosorbent assay (ELISA) detects immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies against the whole spike protein and its receptor binding domain. More than half a million tests have been freely distributed to health institutions in Argentina.
Antibodies were detected in at least 34% of patients within seven days, and in 95% of patients within 45 days of symptom onset. Overall, antibody responses in asymptomatic cases varied widely but were generally lower than those of symptomatic patients. The researchers also developed standardized protocols that were used to assess IgG antibody levels to select suitable blood samples from donors who have recovered from COVID-19 for therapeutic use and clinical trials across the country. Using this protocol, approximately 80% of donor blood samples were potentially suitable for therapies. According to the authors, the study offers a powerful tool for detecting asymptomatic cases and finding better control measures for the pandemic.
The authors add, “The COVIDAR program has built bridges among basic virology researchers, clinical investigators, health care workers, non-profit organizations and policy makers in Argentina for coping with the pandemic. Hopefully, this transformative lesson will help establish collaborative models and priorities for improving public health.”
###
Peer-reviewed; Experimental study; People
In your coverage please use this URL to provide access to the freely available article in PLOS Pathogens:
http://journals.
Citation: Ojeda DS, Gonzalez Lopez Ledesma MM, Pallares HM, Costa Navarro GS, Sanchez L, Perazzi B, et al. (2021) Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog 17(1): e1009161.
https:/
Funding: The COVIDAR project was funded by the CONICET through the Fondo para la Convergencia Estructural del Mercosur (FOCEM), NIH (NIAID) R01.AI095175 and Agencia Argentina de Promoción Científica y Tecnológica PICT2017-1717 Annex COVID-19 to AVG. Founds were also provided by Fundación Williams and Asociación Civil SAND to AVG for COVIDAR and Serokit production and distribution. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
Media Contact
Andrea Gamarnik
[email protected]
Related Journal Article
http://dx.




