New technique models brain cells in older patients more accurately than ever before
Credit: Salk Institute
LA JOLLA–(April 27, 2021) Despite the prevalence of Alzheimer’s, there are still no treatments, in part because it has been challenging to study how the disease develops. Now, scientists at the Salk Institute have uncovered new insights into what goes awry during Alzheimer’s by growing neurons that resemble–more accurately than ever before–brain cells in older patients. And like patients themselves, the afflicted neurons appear to lose their cellular identity.
The findings, published April 27, 2021, in the journal Cell Stem Cell, showed that these brain cells are characterized by markers of stress as well as changes in which the cells become less specialized. Interestingly, many of the alterations seen in these cells are similar to what’s been observed in cancer cells–another disease linked to aging.
“We know the risk of Alzheimer’s increases exponentially with age, but due to an incomplete understanding of age-dependent pathogenesis, it’s been difficult to develop effective treatments,” says Professor and Salk President Rusty Gage, the paper’s senior author. “Better models of the disease are vital for getting at the underlying drivers of this relationship.”
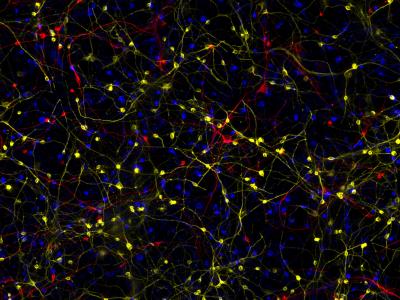
In an earlier study, the Gage lab had shown a new way that skin samples can be used to create brain cells. These induced neurons more accurately reflect the age of the person they came from (unlike neurons made from the more commonly used induced pluripotent stem cells). The new study builds on that finding and is the first to use skin cells from people with Alzheimer’s to create induced neurons that have the characteristics of neurons found in patients’ brains.
“The vast majority of Alzheimer’s cases occur sporadically and have no known genetic cause,” says Jerome Mertens, an assistant adjunct professor at Salk and first author of the paper, who was also involved in that earlier work. “Our goal here was to see if induced neurons that we generated from Alzheimer’s patients could teach us anything new about the changes that take place in these cells when the disease develops.”
In the current research, the investigators collected skin cells from 13 patients with sporadic, age-related Alzheimer’s. They also used cells from three people who have the more rare, inherited form of the disease. As a control, they collected skin cells from 19 people who were matched for age but did not have Alzheimer’s. Using a specialized type of skin cells called fibroblasts, they generated induced neurons from each of the cell donors. They then compared the molecular differences in the cells among those who had Alzheimer’s to the cells of those who didn’t.
The investigators found that the induced neurons made from the cells of people with Alzheimer’s had distinct characteristics that were different from the healthy control subjects’ cells. For one thing, the Alzheimer’s cells had a lack of synaptic structures, which are important for sending signals to each other. They also had changes in their signaling pathways, which control cell function, indicating that the cells were stressed. Additionally, when the researchers analyzed the cells’ transcriptomes–a type of analysis that shows what proteins the cells are making–they found the induced Alzheimer’s neurons had very similar molecular signatures to immature nerve cells found in the developing brain.
According to Mertens, who is also an assistant professor at the University of Innsbruck in Tyrol, Austria, the neurons seem to have lost their mature identity, and this de-differentiation, in which cells lose their specialized characteristics, has also been described in cancer cells. He suggests the finding opens up the door for new studies.
“While more research is needed, the changes associated with the transformation of these cells represent potential targets for therapeutics,” Gage adds.
###
Other authors on the study were Joseph R. Herdy, Larissa Traxler, Simon T. Schafer, Lena Bo?hnke, Dylan A. Reid, Hyungjun Lee, Dina Zangwill, Diana P. Fernandes, Ravi K. Agarwal, Raffaella Lucciola, Shani Stern, and Apua C. M. Paquola of Salk; Johannes C.M. Schlachetzki, Christopher K. Glass, Shauna H. Yuan, Lawrence S.B. Goldstein, and Douglas Galasko of the University of California San Diego (UCSD); Lucia Zhou-Yang, Lukas Karbacher, and Frank Edenhofer of the University of Innsbruck; Steve Horvath of the University of Haifa in Israel; Manching Ku of the University of Freiburg in Germany; and Attila Szu?cs of Eötvös Loránd University in Hungary.
This work was funded by EU ERC-STG-2019-852086, H2020-MSCA-IF- 2017-797205; the BrightFocus Foundation; NIA K99-AG056679; the Chen Foundation; the Austrian FWF-I5057; the AHA-Allen Initiative award 19PABH134610000; the Paul G. Allen Family Foundation; the NIA R01s AG056306, AG056511, and AG057706; the JPB Foundation; the Leona M. and Harry B. Helmsley Charitable Trust; Annette C. Merle-Smith; the G. Harold & Leila Y. Mathers Charitable Foundation; the Ray and Dagmar Dolby Family Fund; the Stichting A.S.C Academy; CIRM RT2-01927; the Austrian FWF-SPIN; the Alzheimer’s Association Research Fellowship; the Alzheimer Nederland Foundation; the DFG-SFB1160-IMPATH; the German Academic Exchange Service DAAD; the Zuckerman STEM leadership program; the Austrian Marshall Plan Foundation; the Dr. Otto Seibert-Foundation; the EU JPND MADGIC through the Austrian BMBWF; the Hungarian ANN-135291; and the Shiley-Marcos Alzheimer’s Disease Research Center at UCSD.
About the Salk Institute for Biological Studies:
Every cure has a starting point. The Salk Institute embodies Jonas Salk’s mission to dare to make dreams into reality. Its internationally renowned and award-winning scientists explore the very foundations of life, seeking new understandings in neuroscience, genetics, immunology, plant biology and more. The Institute is an independent nonprofit organization and architectural landmark: small by choice, intimate by nature and fearless in the face of any challenge. Be it cancer or Alzheimer’s, aging or diabetes, Salk is where cures begin. Learn more at: salk.edu.
Media Contact
Salk Communications
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




