Credit: Rojo Rakotoharisoa
A group of researchers at the University of Ottawa has been looking for ways to improve enzyme design methodologies and recently published their findings in Nature Communications.
Enzymes are used in many industrial and biotechnological applications. With their numerous beneficial properties, they are the most efficient catalysts known – they even have the power to accelerate chemical reactions by more than a billion times. But since the number of naturally occurring enzyme activities is limited, the number of applications also remains limited. While researchers have succeeded in creating artificial enzymes, their catalytic efficiency doesn’t reach the same level as that of natural enzymes.
We talked to senior author Roberto Chica, Full Professor in the Department of Chemistry and Biomolecular Sciences at the University of Ottawa, to learn more about his findings.
Can you please tell us more about artificially designed enzymes?
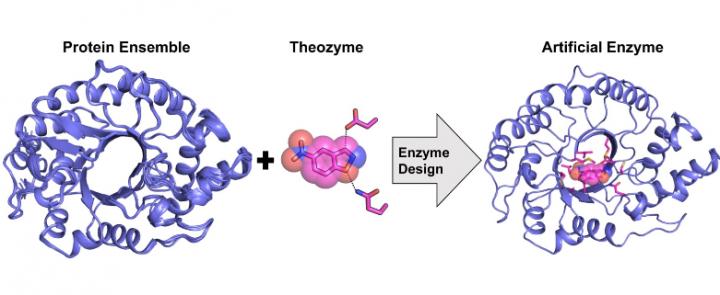
“Over the past 20 years, researchers have successfully designed artificial enzymes from scratch for a variety of model organic transformations. This was done using a procedure called ‘computational enzyme design’ where a catalytic site was computationally built onto a pre-existing protein scaffold devoid of the target catalytic activity.
While successful, this approach has exclusively yielded artificial enzymes displaying catalytic efficiencies that are orders of magnitude lower than those of natural enzymes, requiring subsequent optimization using what is called ‘directed evolution’ to improve activity. Directed evolution is a process whereby random mutations are introduced into a protein to generate a large library of mutant enzymes, which are then screened to identify beneficial mutations. It often requires multiple rounds of random mutagenesis and screening to increase activity significantly.”
How does your research relate to directed evolution?
“In our work, we reveal how directed evolution improves the catalytic efficiency of a computationally designed biocatalyst by approximately 1000-fold by tuning the ensemble of structural sub-states that the enzyme can sample to favor those that are catalytically competent.
Based on these observations, we engineer an artificial biocatalyst with a catalytic efficiency on par with that of the average natural enzyme.”
What is the impactful discovery?
“We developed a novel computational procedure for enzyme design that is more accurate than previous methods because it allows to approximate the intrinsic flexibility of the protein scaffold used as a template for design.”
Why is this important?
“This is important because previous methods focused on creating a stable structure that ignores the inherent dynamism in natural enzymes, which is crucial to their function (i.e. enzymes must “move” to be efficient catalysts).
Previously, it was not known whether an artificial enzyme displaying a catalytic efficiency on par with that of a natural enzyme could be computationally designed. We show that this is possible but only by using a structural ensemble of protein templates approximating conformational flexibility instead of a single template as previously done.
The results presented in our manuscript suggest that computational enzyme design using a structural ensemble could prevent the need for directed evolution by allowing catalytically competent sub-states to be sampled during the design procedure.”
What are the potential applications of your research?
“If we could design, from scratch, enzymes that can catalyze any target chemical reaction with high efficiency, it would open the door to highly valuable biotechnologies that are currently inaccessible using natural enzymes.”
Is there anything you’d like to add?
Yes, research took place from 2018 to 2020, at the University of Ottawa and the University of California, San Francisco.
###
uOttawa PhD student, Rojo V. Rakotoharisoa, and former uOttawa postdoctoral fellow, Aron Broom, are the lead authors of this study that was done in collaboration with the group of James S. Fraser, from the Department of Bioengineering and Therapeutic Science at UC San Francisco.
The paper “Ensemble-based enzyme design can recapitulate the effects of laboratory directed evolution in silico” was recently published in Nature Communications.
Media Contact
Justine Boutet
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




