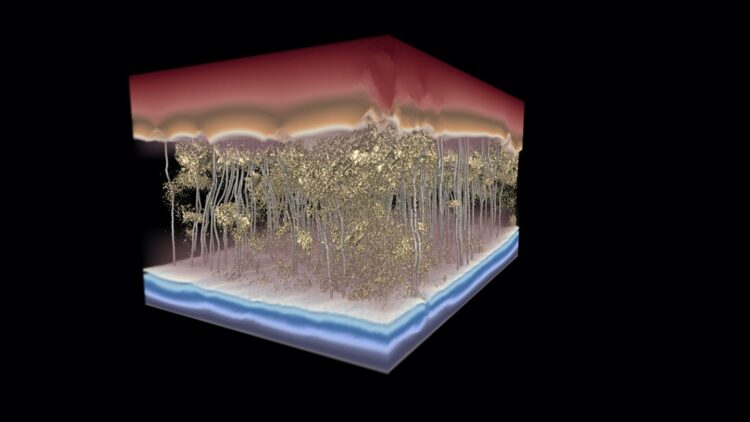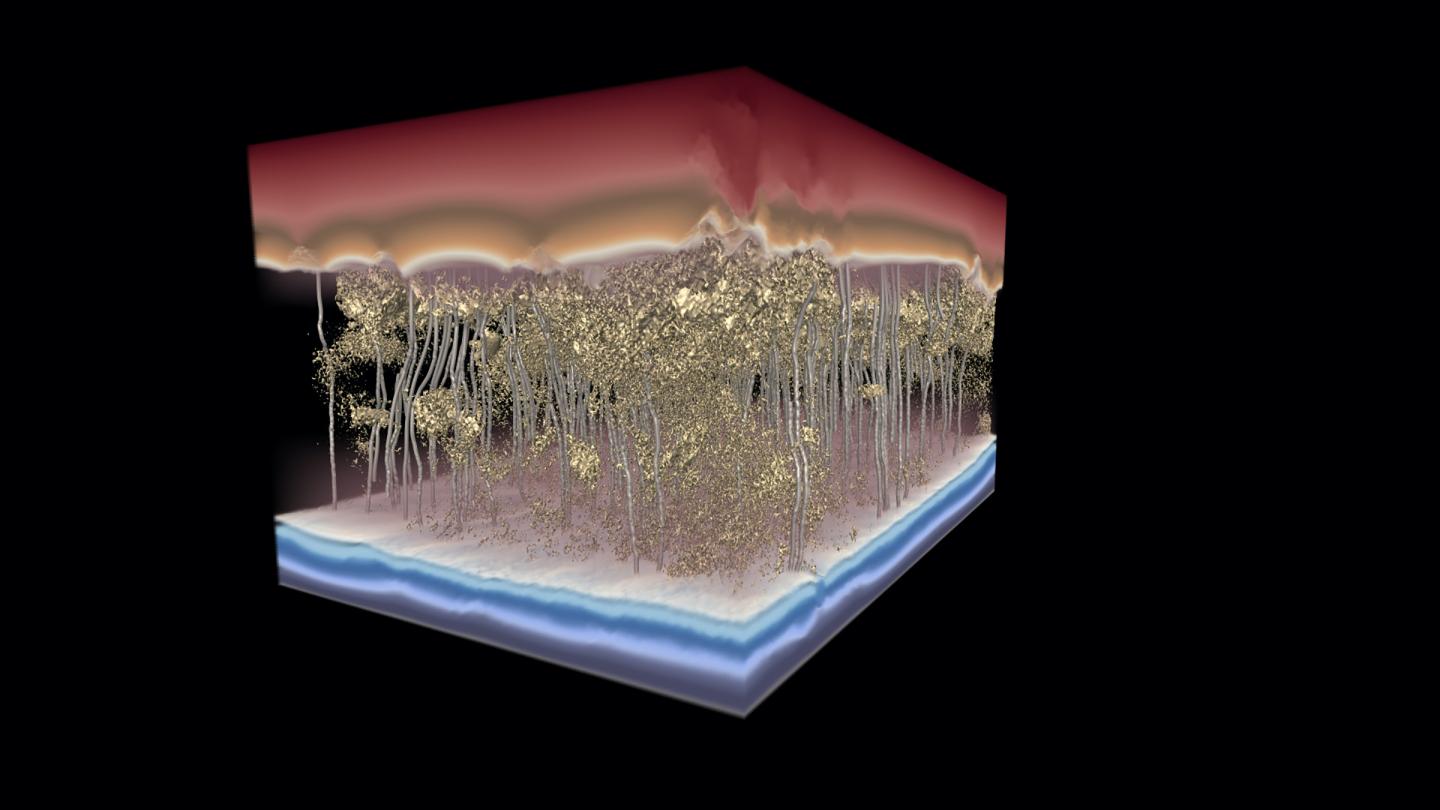
Credit: Image by the Ganapathysubramanian research group/Iowa State University and Gregory Foss/Texas Advanced Computing Center.
AMES, Iowa – Nature has figured out how to make great membranes.
Biological membranes let the right stuff into cells while keeping the wrong stuff out. And, as researchers noted in a paper just published by the journal Science, they are remarkable and ideal for their job.
But they’re not necessarily ideal for high-volume, industrial jobs such as pushing saltwater through a membrane to remove salt and make fresh water for drinking, irrigating crops, watering livestock or creating energy.
Can we learn from those high-performing biological membranes? Can we apply nature’s homogenous design strategies to manufactured, polymer membranes? Can we quantify what makes some of those industrial membranes perform better than others?
Researchers from Iowa State University, Penn State University, the University of Texas at Austin, DuPont Water Solutions and Dow Chemical Co. – led by Enrique Gomez of Penn State and Manish Kumar of Texas – have used transmission electron microscopy and 3D computational modeling to look for answers.
Iowa State’s Baskar Ganapathysubramanian, the Joseph C. and Elizabeth A. Anderlik Professor in Engineering from the department of mechanical engineering, and Biswajit Khara, a doctoral student in mechanical engineering, contributed their expertise in applied mathematics, high-performance computing and 3D modeling to the project.
The researchers found that creating a uniform membrane density down to the nanoscale of billionths of a meter is crucial for maximizing the performance of reverse-osmosis, water-filtration membranes. Their discovery has just been published online by the journal Science and will be the cover paper of the Jan. 1 print edition.
Working with Penn State’s transmission electron microscope measurements of four different polymer membranes used for water desalination, the Iowa State engineers predicted water flow through 3D models of the membranes, allowing detailed comparative analysis of why some membranes performed better than others.
“The simulations were able to tease out that membranes that are more uniform – that have no ‘hot spots’ – have uniform flow and better performance,” Ganapathysubramanian said. “The secret ingredient is less inhomogeneity.”
Just take a look at the Science cover image the Iowa State researchers created with assistance from the Texas Advanced Computing Center, said Khara: Red above the membrane shows water under higher pressure and with higher concentrations of salt; the gold, granular, sponge-like structure in the middle shows denser and less-dense areas within the salt-stopping membrane; silver channels show how water flows through; and the blue at the bottom shows water under lower pressure and with lower concentrations of salt.
“You can see huge amounts of variation in the flow characteristics within the 3D membranes,” Khara said.
Most telling are the silver lines showing water moving around dense spots in the membrane.
“We’re showing how water concentration changes across the membrane.” Ganapathysubramanian said of the models which required high-performance computing to solve. “This is beautiful. It has not been done before because such detailed 3D measurements were unavailable, and also because such simulations are non-trivial to perform.”
Khara added, “The simulations themselves posed computtional challenges, as the diffusivity within an inhomogeneous membrane can differ by six orders of magnitude”
So, the paper concludes, the key to better desalination membranes is figuring out how to measure and control at very small scales the densities of manufactured membranes. Manufacturing engineers and materials scientists need to make the density uniform throughout the membrane, thus promoting water flow without sacrificing salt removal.
It’s one more example of the computational work from Ganapathysubramanian’s lab helping to solve a very fundamental yet practical problem.
“These simulations provided a lot of information for figuring out the key to making desalination membranes much more effective,” said Ganapathysubramanian, whose work on the project was partly supported by two grants from the National Science Foundation.
###
The research team
The project was led by Enrique Gomez, a professor of chemical engineering and materials science and engineering at Penn State University, and Manish Kumar, an associate professor of civil, architectural and environmental engineering at the University of Texas at Austin.
Also, from Iowa State University: Biswajit Khara, Baskar Ganapathysubramanian; from Penn State: Tyler Culp, Kaitlyn Brickey, Michael Geitner, Tawanda Zimudzi, Andrew Zydney; from DuPont Water Solutions: Jeffrey Wilbur, Steve Jons; and from Dow Chemical Co.: Abhishek Roy, Mou Paul.
Media Contact
Baskar Ganapathysubramanian
[email protected]
Related Journal Article
http://dx.