The finding provides a surrogate for testing the effectiveness of vaccine candidates

Credit: Jennifer Jenks, Duke University School of Medicine
DURHAM, N.C. — A Duke Health-led research team has identified a key marker that will help speed effective vaccine designs for cytomegalovirus (CMV), the most common congenital infection worldwide and a leading cause of infant brain damage.
In a study appearing online Nov. 4 in Science Translational Medicine, the researchers describe an immune surrogate that demonstrates when a vaccine has elicited the necessary antibodies that protect against CMV infection. The finding is already being applied to screen potential vaccines.
“CMV has been recognized as a top priority for vaccine development for more than 20 years, yet we remain without an approved vaccine. This work provides a way to assure that current and future vaccine candidates stimulate an effective immune response,” said senior author Sallie Permar, M.D., a professor in the departments of Pediatrics, Immunology, Molecular Genetics and Microbiology, and Pathology at Duke University School of Medicine.
“We are beyond due for vaccines to be developed to protect against this virus, which infects 40,000 infants a year in the United States alone, with a third of these children developing permanent hearing loss, brain damage or neuro-developmental delays,” Permar said.
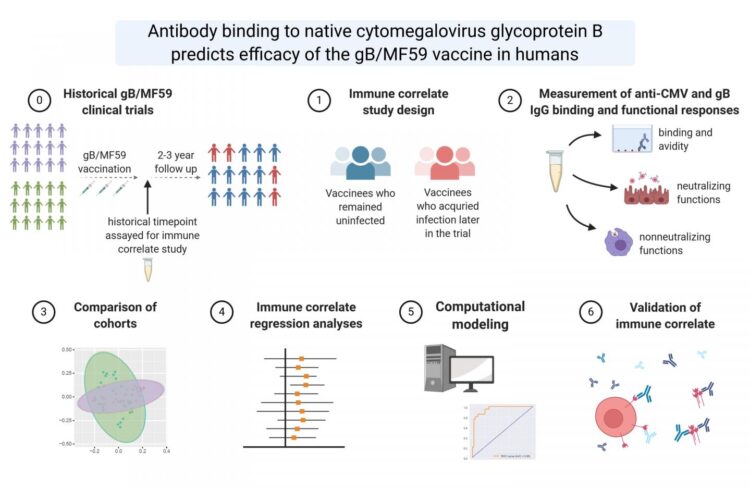
Permar and colleagues, including lead author Jennifer A. Jenks, an M.D./Ph.D. candidate at Duke, investigated the immune responses that protected against CMV infections in women who received the investigational protein vaccine gB/MF59. The main component of this vaccine was the CMV protein “gB,” which the virus uses to enter human cells.
The investigational vaccine was expected to generate an immune response that could stop CMV from entering host cells. It was about 50-percent effective in preventing CMV infection in multiple Phase 2 clinical trials, but an acceptable CMV vaccine should be at least 70-percent effective.
The researchers found that protection against CMV infection was associated with the presence of antibodies in the blood that bind to the target protein gB when it is presented on a cell surface, but not to gB when it is in its soluble, free-floating form used in the gB/MF59 vaccine. This finding suggests that future CMV vaccines should be designed to target the appropriate conformation of gB. Additionally, the researchers report, the presence of these antibodies may be used to predict the potential efficacy of future candidate vaccines.
“This is an important immunologic endpoint for vaccine development and evaluation,” Jenks said. “This could serve as a surrogate for assessing antiviral function and could aid in vaccine evaluation in preclinical and early-phase clinical trials.”
###
In addition to Permar and Jenks, study authors include Cody S. Nelson, Hunter K. Roark, Matthew L. Goodwin, Robert F. Pass, David I. Bernstein, Emmanuel B. Walter, Kathryn M. Edwards, Dai Wang, Tong-Ming Fu, Zhiqiang An and Cliburn Chan.
Media Contact
Sarah Avery
[email protected]