Credit: © 2021 KAUST
Exploiting the unusual metal-reducing ability of the iron-breathing bacterium Geobacter sulfurreducens, KAUST researchers have demonstrated a cheap and reliable way to synthesize highly active single-atom catalysts. The innovation, which could dramatically improve the efficiency and cost of hydrogen production from water, highlights the role nature can play in the search for new energy systems.
Many chemical reactions require a catalyst as a reactive surface where atoms or molecules are brought together with the right amount of energy to spark a chemical change. Water, for example, can be split into hydrogen and oxygen atoms by reacting on a pair of electrodes made of platinum and iridium oxide. The efficiency of the reaction, however, depends largely on how many atoms can get involved.
“In a nanoparticle catalyst, only 20 percent of the metal atoms might be available for catalysis,” says Srikanth Pedireddy, formerly at KAUST and now at the University of Exeter, U.K. “Single-atom catalysts, on the other hand, allow 100 percent atomic utilization and so are promising for various catalyst applications; however, conventional synthesis methods are expensive, involve high temperatures and only give low yields with poor atomic distribution.”
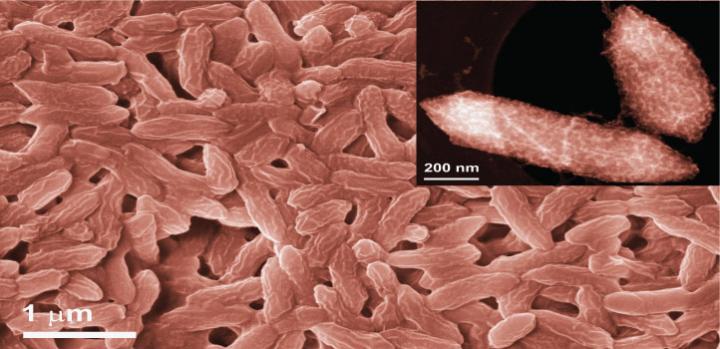
In search of a more reliable and cost-effective approach, Pedireddy, Pascal Saikaly and their colleagues turned to nature. The anaerobic bacterium G. sulfurreducens is unusual in that it “breathes” iron, not oxygen, and has the remarkable ability to conduct electrons from inside to outside the cell.
“This bacterium has redox active proteins called c-type cytochromes that contain a heme complex — a central iron atom coordinated to four nitrogen atoms of a porphyrin ring,” says Pedireddy. “We envisaged that this heme site could be used to chemically reduce single atoms of catalytically active metals instead of iron.”
After confirming the formation of single iron atoms at the cytochrome sites on the surface of bacterial cells, the team immersed the bacteria in a solution containing iridium, which produced a similar and very satisfying result.
“Seeing single atoms on the surface of bacteria was a major challenge,” says Pedireddy. “With the high-resolution electron microscopy facilities at KAUST, we were able to visualize the atomically dispersed single atoms of metals on the bacteria surface.”
The team found they could load the bacteria with up to 1 percent of well-dispersed single-atom iridium, giving a more reliable catalyst with comparable hydrogen-evolving activity to the platinum/carbon standard at a fraction of the cost of other single-atom methods.
“Our work could inspire the use of other efficient electroactive bacteria for synthesizing high-performing and low-cost electrocatalysts for various energy-related applications,” Saikaly says.
###
Media Contact
Michael Cusack
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




