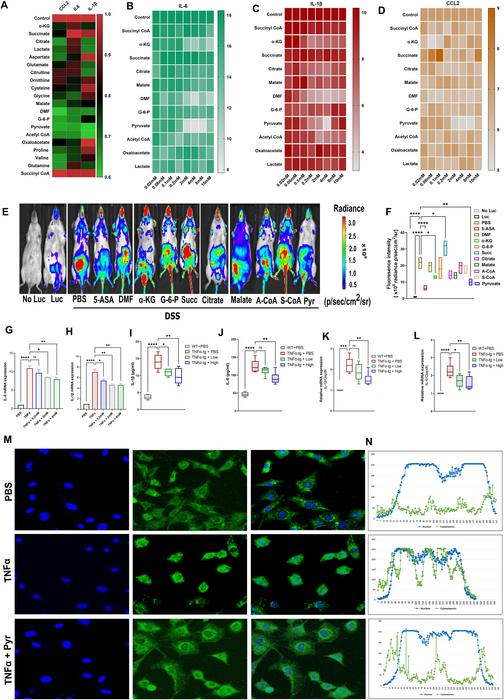
In a remarkable scientific advancement, researchers have unveiled the therapeutic potential of exogenously administered pyruvate in the treatment of ulcerative colitis (UC), a chronic and debilitating inflammatory bowel disease. This revelation, detailed in a recent publication in the journal Genes & Diseases, elucidates how pyruvate exerts its anti-inflammatory effects by targeting the cytosolic phospholipase A2 (cPLA2) enzyme. The findings herald a promising new frontier in UC therapeutics, challenging the current paradigms dominated by biologics and immunosuppressants.
Ulcerative colitis, characterized by persistent inflammation of the colonic mucosa, manifests clinically through severe abdominal discomfort, frequent diarrhea, rectal bleeding, and systemic manifestations such as weight loss and fatigue. Existing pharmacotherapies, including aminosalicylic acids, corticosteroids, and immunosuppressive agents, while standard, are often burdened with significant adverse effects and variable efficacy. Notably, anti-TNFα biological agents, though effective, suffer from limitations such as parenteral administration and high cost, sparking the urgent need for novel, orally available therapeutics with favorable safety profiles.
The investigative team approached this challenge by conducting comprehensive in vitro screenings of numerous cellular metabolites to identify candidates capable of modulating the pro-inflammatory TNFα/NF-κB signaling axis known to drive intestinal inflammation in UC. Pyruvate emerged as a standout molecule, exhibiting robust inhibition of TNFα-mediated NF-κB activation. Subsequent in vivo validation using dextran sodium sulfate (DSS)-induced colitis mouse models demonstrated that oral pyruvate administration markedly ameliorated colonic inflammation, preserved mucosal architecture, and improved clinical indices of disease severity.
.adsslot_l0Y7PMGifF{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_l0Y7PMGifF{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_l0Y7PMGifF{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Histopathological analyses underscored pyruvate’s capacity to maintain intestinal epithelial barrier integrity, a critical factor often compromised in UC pathogenesis. Examination of tight junction components revealed that pyruvate restored expression of proteins including claudin-2, ZO-1, and occludin, fortifying the mucosal barrier against disruptive inflammatory insults. Consequently, pyruvate’s mechanism of action extends beyond mere immunomodulation, encompassing preservation of epithelial homeostasis.
Delving deeper into molecular mechanisms, the researchers pinpointed cytosolic phospholipase A2 (cPLA2) as a novel and essential pyruvate target. Employing sophisticated assays such as drug affinity responsive target stability (DARTS) and cellular thermal shift assays (CETSA), they demonstrated that pyruvate directly binds to and stabilizes cPLA2, shielding it from proteolytic degradation. This biochemical interaction is pivotal given that cPLA2 catalyzes the release of arachidonic acid, a precursor for a suite of pro-inflammatory lipid mediators implicated in NF-κB pathway activation and immune cell recruitment.
Functionally, the binding of pyruvate to cPLA2 attenuates the downstream cascade of inflammatory signaling, culminating in substantial reductions in key cytokines like interleukin-1β (IL-1β) and interleukin-6 (IL-6) within both systemic circulation and colonic tissue. The dampening of these cytokines is crucial, as they orchestrate the amplification and perpetuation of mucosal inflammation characteristic of UC. Importantly, these anti-inflammatory effects were abrogated in cPLA2-deficient murine models, confirming the indispensable role of cPLA2 as the mediator of pyruvate’s therapeutic efficacy.
The researchers also speculated on the broader implications of their findings. Given the centrality of the TNFα/NF-κB axis not only in UC but also in a plethora of inflammatory and autoimmune diseases, pyruvate’s modulatory action on cPLA2 could translate into therapeutic benefits across a spectrum of TNFα-associated disorders. This opens avenues for exploring pyruvate-based interventions in rheumatoid arthritis, psoriasis, and even certain neuroinflammatory conditions where aberrant NF-κB signaling exacerbates pathology.
The study’s multi-tiered approach, integrating cellular assays, murine disease models, and molecular mechanistic evaluations, underscores the robust scientific foundation underpinning the therapeutic promise of pyruvate. Additionally, the elucidation of pyruvate’s effect on tight junction proteins aligns with emerging paradigms that emphasize restoration of epithelial barrier function as a critical therapeutic endpoint in inflammatory bowel diseases.
Moreover, the methodological rigor surrounding the identification of cPLA2 as the direct molecular target was facilitated by advanced biochemical techniques adept at capturing protein-ligand interactions in a cellular context. The stability shifts in cPLA2 upon pyruvate binding highlight a nuanced regulatory mechanism, wherein metabolic intermediates can directly influence enzyme activity and degradation, adding a new dimension to metabolic regulation of inflammation.
Given the chronic and relapsing nature of UC, therapies that combine efficacy with tolerability and patient convenience are urgently needed. Pyruvate’s oral availability, coupled with its dual action on immune signaling and barrier stabilization, could revolutionize the clinical management of UC. The researchers advocate for the advancement of pyruvate into clinical trials to verify its safety and effectiveness in human subjects, which could ultimately transform therapeutic strategies for millions worldwide burdened by inflammatory bowel diseases.
In sum, this study delineates a compelling narrative wherein exogenous pyruvate emerges not merely as a metabolic substrate but as a potent anti-inflammatory agent targeting the vital enzyme cPLA2, thereby disrupting the deleterious TNFα/NF-κB signaling pathway. This dual action framework offers a fresh, scientifically grounded hope for developing safer, more effective, and orally administered therapies for ulcerative colitis and potentially other chronic inflammatory conditions.
Subject of Research: Ulcerative colitis treatment via modulation of inflammatory pathways through metabolic intermediates
Article Title: Exogenous pyruvate is therapeutic against colitis by targeting cytosolic phospholipase A2
References:
Sadaf Hasan, Nabil Ghani, Xiangli Zhao, Julia Good, Chuan-ju Liu, Exogenous pyruvate is therapeutic against colitis by targeting cytosolic phospholipase A2, Genes & Diseases, 2025, 101571.
Image Credits: Genes & Diseases
Keywords: ulcerative colitis, pyruvate, cytosolic phospholipase A2, TNFα, NF-κB signaling, inflammatory bowel disease, tight junction proteins, IL-1β, IL-6, arachidonic acid, DSS-induced colitis, oral therapy
Tags: anti-inflammatory effects of pyruvatebiologics and immunosuppressants limitationscellular metabolites in UCchronic intestinal inflammation therapycomprehensive in vitro screeningscytosolic phospholipase A2 inhibitioninflammatory bowel disease researchnovel oral therapeutics for UCpromising new frontiers in UC therapeuticspyruvate therapeutic potentialTNFα/NF-κB signaling modulationulcerative colitis treatment