Credit: Tatsuo Suzuki, Shinshu University
[Outline]
A research group led by Specially appointed professor, Dr. Tatsuo Suzuki of Shinshu University School of Medicine developed a new purification protocol for Postsynaptic density (PSD) lattice, a core structure of the PSD of excitatory synapses in the central nervous system. The components of the PSD lattice were identified by comprehensive shotgun mass spectrometry and categorized as either minimum essential component (MEC) or non-MEC proteins. Tubulin was found to be a major component of the MEC, with non-microtubule tubulin widely distributed on the purified PSD lattice. The presence of tubulin in and around PSDs was verified by post-embedding immuno-gold labeling EM of cerebral cortex. This study newly identified the PSD lattice backbone structure and provides a new PSD architecture model comprising a non-microtubule tubulin-based backbone structure and its associated proteins, including various PSD scaffold/adaptor proteins and other PSD proteins.
[Background]
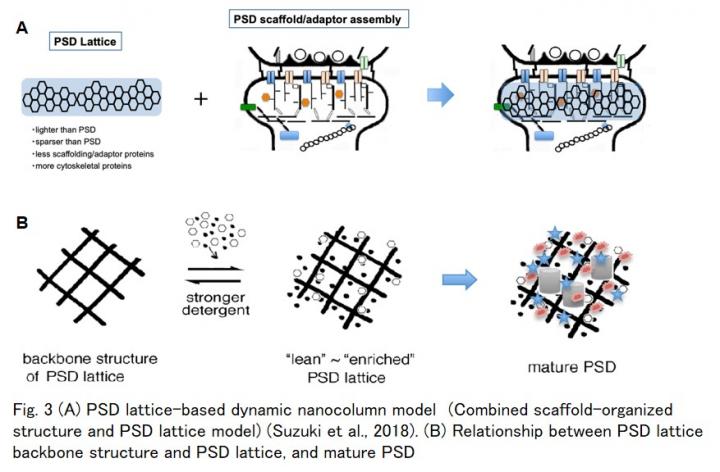
Structural changes in postsynaptic density (PSD) (Fig.1A) are important mechanisms for maintaining synaptic plasticity, the basis for memory and learning. The molecular mechanism underlying changes in PSD structure (PSD remodeling) is currently unknown. A complete understanding of the structure of PSD is indispensable to fully elucidate the molecular mechanisms of spine and PSD dynamics during the expression of synaptic plasticity. Currently, it is tacitly believed that PSD is formed as an assembly of various kinds of scaffold/adaptor proteins (Scaffold/adaptor assembly model) (Fig. 1B right). On the contrary, the PSD lattice meshwork structure was found to be buried in the PSD by EM observation as early as the 1970’s. However, major constituents of the PSD lattice and the relationship between the PSD lattice and the scaffold protein assembly model, remain unknown (Fig. 1B). In a previous study, the same research group purified a PSD lattice and proposed “PSD lattice-based dynamic nanocolumn” model (Fig. 3A) for the molecular architecture of PSD, in which the scaffold protein model and the PSD lattice model are combined (Suzuki et al., 2018). However, the group was unable to elucidate the molecular components of the PSD lattice due to the insolubility of the protein components.
[The key results of this research]
1) New purification protocol of PSD lattice was established.
2) PSD lattice backbone structure was newly identified (Fig. 3B).
3) Tubulin was by far the most common component of the PSD lattice backbone structure and widely distributed on the purified PSD lattice structure (Fig. 2A).
4) Tubulin associated with the PSD lattice backbone structure is in a non-microtubule form (Fig. 2A).
5) Postembedding immuno-gold EM observation using anti-tubulin antibody confirmed the presence of tubulin-immunoreactivity in and around PSD in the mouse brain (Fig. 2B).
6) Only a minimum amount of typical PSD scaffold/adaptor proteins are associated with PSD lattice backbone structure.
[Conclusion from the results and significance of this study]
This study proposes a PSD lattice model comprising a non-microtubule tubulin-based backbone structure and its associated proteins, including various PSD scaffold/adaptor proteins and other PSD proteins. (Fig. 3B) (This is the 2nd version of our PSD architecture model proposed in 2018.)
PSD lattice backbone structure is different from the three-dimensional assembly constructed by postsynaptic scaffold/adaptor proteins (Fig. 3B).
In the new model, non-microtubule tubulin may play an important role as a platform to which PSD scaffold/adaptor proteins and various PSD-functioning molecules become associated while synapses mature and reorganize (Fig. 3B).
[Future research prospects]
1) Elucidation of the mechanism of how non-microtubule type tubulin is related with PSD lattice backbone is important.
2) Role of PSD lattice other than essential cytoskeleton for the PSD should be searched.
3) Mechanisms of synaptogenesis and synaptic plasticity involving PSD lattice are important research targets.
###
[Article]
The results will be published in the international, open-access journal Life Science Alliance on May 18th.
Suzuki T., et al.
Non-microtubule tubulin-based backbone and subordinate components of postsynaptic density lattices
Life Science Alliance (URL: https:/
DOI: 10.26508/lsa.202000945
[Related article]
T. Suzuki, K. Kametani, W. Guo, and W. Li. 2018. Protein components of post-synaptic density lattice, a backbone structure for type I excitatory synapses. J Neurochem. 144:390-407.
DOI: 10.1111/jnc.14254
[Terminology]
Synapse, spine, and PSD: For location in the neuronal cells, see Fig. 1A.
Postsynaptic density (PSD):
PSD is a specialized cytoskeleton localized immediately underneath the postsynaptic membrane of the nerve cells. PSD functions as an essential device for synaptic transmission and plasticity and is critical for normal synaptic and brain activities. PSD is not just a signal-processing machinery that mediates synaptic transmission but is also a “synaptic plasticity generator” that can change its own properties to affect efficacies of synaptic transmission. PSD is not a static cytoskeleton but a dynamic structure, changing its properties (size, shape, composition, etc) depending on synaptic inputs.
PSD lattice:
Network structure, identified in EM observation by extraction of synaptic plasma membrane or TritonX-100-insoluble PSD with relatively strong detergent, deoxycholate (DOC), and proposed to be an underlining structure of the PSD (Matus and Walters, 1975; Blomberg et al. 1977; Matus and Taff-Jones 1978; Matus 1981).
Tubulin, microtubule in and around PSD:
The presence of tubulin in the in situ PSD seems to have not yet been widely accepted possibly because there had been no reports on the detailed distribution of tubulin in the synaptic region of the brain using immuno-gold labeling. This might be owing to the sensitive nature of tubulin to nearby conditions. Similar instability is also observed in microtubules, a fibrous cytoskeleton formed with polymerized tubulin, in spine head, in which microtubule appears only transiently.
2021.5.13
By a group led by Specially Appointed Professor Dr. Tatsuo Suzuki of Shinshu University School of Medicine.
Media Contact
Hitomi Thompson
[email protected]
Related Journal Article
http://dx.




