Scientists highlight advances and discuss future prospects for the use of organoid systems (‘organs in a dish’) in gastroenterology and hepatology in Digestive and Liver Disease
Credit: Digestive and Liver Disease
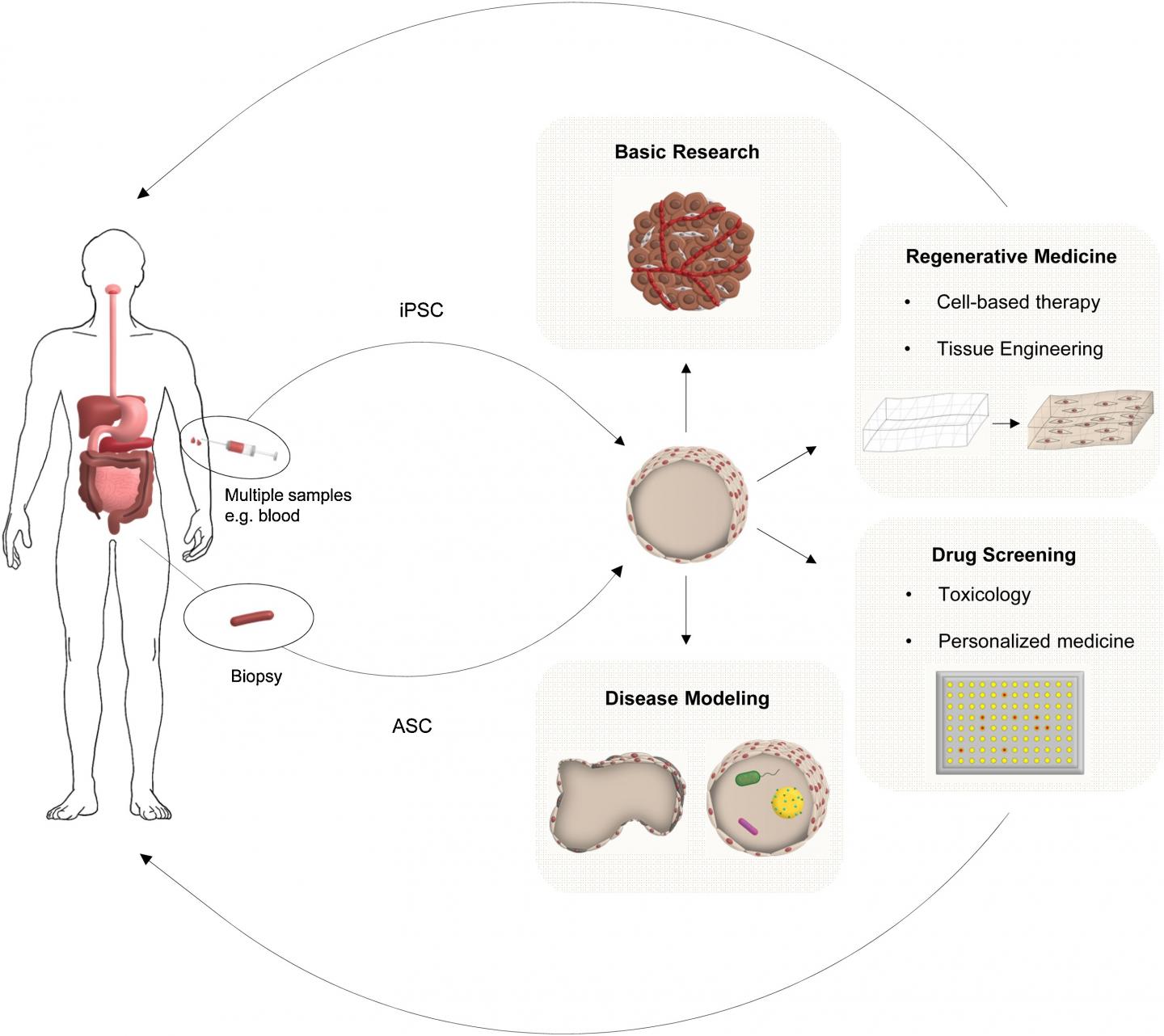
Oxford, June 3, 2019 – One of the most exciting advancements in stem cell research has been the development of organoid systems, which are organ-like three-dimensional structures that mimic their corresponding organ in vivo. In this important review in Digestive and Liver Disease, published by Elsevier, scientists highlight some of the established and exciting novel uses for organoids or “organs in a dish” in gastroenterology and hepatology and look towards the future in this exciting field.
Recent advances in stem cell biology have enabled long-term culturing of organotypic intestinal or hepatic tissues derived from tissue resident or pluripotent stem cells. These 3D structures, called organoids, represent a substantial advancement in structural and functional complexity over traditional in vitro cell culture models that are often non-physiological and transformed. Organoids can recapitulate the in vivo architecture, functionality, and genetic signature of the corresponding tissue. Therefore, there is increasing interest in using such cultured cells as a source for tissue engineering, regenerative medicine, and personalized medicine.
“Organoid technology can be used to reveal novel insights into basic biology such as stem cell biology, organogenesis, cellular differentiation, cell-cell interaction, and physiological functions but is also important for the future of regenerative medicine,” explained lead author Prof. Dr. Markus F. Neurath, MD, Department of Medicine, University Hospital, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Germany. “They can also be used to study the pathophysiology of various human diseases such as cancer, infection (host-microbe interaction), inflammation, and hereditary diseases such as cystic fibrosis.”
Since organoids serve as a novel tool to model epithelial cell biology, epithelial turnover, barrier dynamics, immune-epithelial communication and host-microbe interaction, organoids have been chosen the method of the year 2017, not only for medical approaches, but also for basic science.
The authors highlight some of the most significant advances made over the past ten years related to gastroenterology and hepatology that have the potential to improve patient outcomes:
- Hepatobiliary organoids were first introduced in 2007 to demonstrate the capacity of bipotent hepatoblasts to commit to a biliary lineage. They were based on spontaneous differentiation and resulted in organoids with limited function and maturity, which provided proof-of-principle for the present generation of hepatobiliary organoids.
- Intestinal organoids were first described in 2009 and can be used to study the pathophysiology of a variety of human diseases including gastrointestinal inflammation such as inflammatory bowel disease (IBD) and celiac disease, infections such as Helicobacter and Salmonella, malignancy, and genetic diseases. Patient-derived organoids enable drug screening and personalized approaches for treating intestinal diseases.
- Studies using epithelial organoids have increased the understanding of the pathophysiology underlying Crohn’s disease, a disease that is still incompletely understood. In 2011, scientists demonstrated that caspase-8 deficient intestinal organoids display increased sensitivity towards a proinflammatory kind of cell death (necroptosis) and additionally an altered Paneth cell homeostasis. Researchers compared the results obtained in the caspase-8 deficient organoid cultures to data derived from primary human tissue and uncovered a significant overlap. These data indicate that organoids are useful for understanding the physiology of Paneth cells in particular, which is of significance since Paneth cell dysfunction is a hallmark of ileal inflammation in Crohn’s disease.
- The ability to grow organoids from healthy murine and human intestinal tissue has paved the way for organoids derived from patient tumor material. These tumor organoids phenotypically and genetically resemble the tumor epithelium from which they are derived, and thus represent a promising tool to potentially enable patient-specific anticancer drug testing.
- In vitro production of bona-fide hepatocytes could provide a unique platform for disease modeling and high-throughput drug screening for liver disease. Recent research has led to successful propagation of primary hepatocytes from fetal or adult whole liver in both humans and mice. The resulting hepatocyte organoids expressed markers and function at levels comparable to primary tissue, providing a promising platform for translational applications such as drug screening. Hepatobiliary organoids have been used to model several monogenic liver diseases including cystic fibrosis, polycystic liver disease, Alagille syndrome, Wilson disease, and alpha1-antitrypsin deficiency. These patient-derived organoids recapitulate the disease phenotype in vitro and can be used as drug screening platforms for the development of novel therapeutics for personalized medicine.
- The treatment of liver disorders is restricted by the shortage of healthy tissue suitable for transplantation. Looking forward, organoids represent a source of functionally mature, easily expandable cells that could address this issue. Experiments in animal models have provided the first proof-of-principle for the feasibility and efficacy of transplantation of hepatobiliary organoids.
“Although gastrointestinal and hepatobiliary organoids have been developed only in the last decade, they have already proven to be an invaluable tool for drug screening, disease modelling, and regenerative medicine,” concluded Prof. Dr. Neurath and co-authors. “Challenges still remain; however, recent advances in bioengineering, organ-on-chip technology, single-cell analyses, and synthetic matrix development hold great promise for addressing current limitations and advancing the field even further, both in terms of mechanistic studies and clinical translation.”
###
Media Contact
Victoria Wetherell
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




