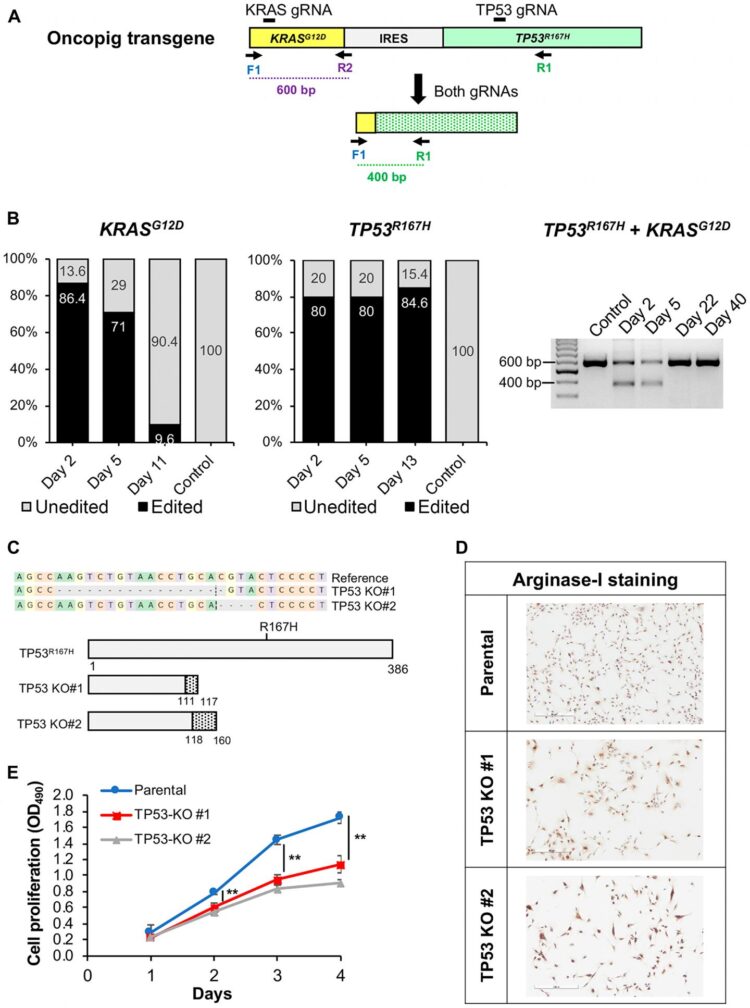
Volume 11, Issue 28 of Oncotarget features “Development and comprehensive characterization of porcine hepatocellular carcinoma for translational liver cancer investigation” by Gaba et, al. which reported that reliable development of Oncopig HCC cell lines
Credit: Kyle M. Schachtschneider – [email protected]
Volume 11, Issue 28 of Oncotarget features “Development and comprehensive characterization of porcine hepatocellular carcinoma for translational liver cancer investigation” by Gaba et, al. which reported that reliable development of Oncopig HCC cell lines was demonstrated through hepatocyte isolation and Cre recombinase exposure across 15 Oncopigs.
Oncopig and human HCC cell lines displayed similar cell cycle lengths, alpha-fetoprotein production, arginase-1 staining, chemosusceptibility, and drug-metabolizing enzyme expression.
The ability of Oncopig HCC cells to consistently produce tumors in vivo was confirmed via subcutaneous injection into immunodeficient mice and Oncopigs.
Reproducible development of intrahepatic tumors in an alcohol-induced fibrotic microenvironment was achieved via engraftment of SQ tumors into fibrotic Oncopig livers.
Finally, Oncopig HCC cells are amenable to gene editing for the development of personalized HCC tumors.
Dr. Kyle M. Schachtschneider from The Department of Radiology and The Biological Resources Laboratory at The University of Illinois at Chicago as well as The National Center for Supercomputing Application at The University of Illinois at Urbana-Champaign said, “Hepatocellular carcinoma (HCC)–the most common type of primary liver cancer–is an aggressive cancer that spans more than 850,000 new yearly diagnoses and causes 800,000 annual deaths, representing the fifth most common cancer globally and the second most common cause of cancer-related death worldwide.“
The rabbit VX2 model has been considered the most relevant and widely used model to test HCC LRTs to date.
As such, there is a crucial need for more clinically relevant large animal models that faithfully recapitulate human HCC to address unmet clinical needs and serve as a bridge between murine studies and clinical practice.
This study describes the utilization of the Oncopig Cancer Model for the development of a clinically relevant, translational porcine HCC model.
The Oncopig Cancer Model is a transgenic pig model that develops site and cell-specific tumors following Cre recombinase induced expression of heterozygous KRASG12D and TP53R167H transgenes.
The large size of the pig and its similarities with humans in terms of anatomy, physiology, metabolism, immunity, and genetics make it an ideal model species for the development of a large animal cancer model.
Development of Oncopig HCC cell lines has been previously described, however, prior work was limited to characterization of HCC cell lines derived from three Oncopigs, minimal in vitro and in vivo profiling, and no description of intrahepatic tumors.
As such, this study was undertaken to test the hypothesis that phenotypically consistent Oncopig HCC cells that faithfully recapitulate the in vitro features of human HCC can be developed across a large Oncopig cohort and that these cells can be utilized to develop clinically relevant intrahepatic HCC tumors in Oncopigs.
The Schachtschneider Research Team concluded in their Oncotarget Research Paper, “the Oncopig HCC model offers a novel, physiologically and anatomically relevant cancer model for which a multitude of innovative therapeutic modalities can be applied and tested while significantly reducing the costs, confounding variables seen in human subjects, and lengthy conduct of human clinical trials. Importantly, the Oncopig can be utilized to conduct correlative studies for more efficient and consistent investigation of new therapies. Its size allows for utilization of the same methods and instruments used in human clinical practice, including CT and magnetic resonance imaging technologies. This model is thus amenable to developing and establishing medical imaging standards related to diagnosing HCC tumors and tracking treatment response using accepted radiologic criteria, a critical facet of therapeutic discovery and validation. Importantly, the Oncopig is also immunocompetent, lending itself to investigation of immunotherapies [32]. Therefore, the Oncopig fulfills the currently unmet clinical modeling needs for HCC, particularly for pilot investigations of experimental therapies or experimental therapeutic combinations not feasible in human subjects.“
“The Oncopig HCC model offers a novel, physiologically and anatomically relevant cancer model for which a multitude of innovative therapeutic modalities can be applied and tested while significantly reducing the costs, confounding variables seen in human subjects, and lengthy conduct of human clinical trials. Importantly, the Oncopig can be utilized to conduct correlative studies for more efficient and consistent investigation of new therapies. Its size allows for utilization of the same methods and instruments used in human clinical practice, including CT and magnetic resonance imaging technologies. This model is thus amenable to developing and establishing medical imaging standards related to diagnosing HCC tumors and tracking treatment response using accepted radiologic criteria, a critical facet of therapeutic discovery and validation. Importantly, the Oncopig is also immunocompetent, lending itself to investigation of immunotherapies [32]. Therefore, the Oncopig fulfills the currently unmet clinical modeling needs for HCC, particularly for pilot investigations of experimental therapies or experimental therapeutic combinations not feasible in human subjects.”
Sign up for free Altmetric alerts about this article
DOI – https:/
Full text – https:/
Correspondence to – Kyle M. Schachtschneider – [email protected]
Keywords –
liver cancer,
transgenic pigs,
large animal model,
interventional radiology,
personalized medicine
About Oncotarget
Oncotarget is a weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https:/
SoundCloud – https:/
Facebook – https:/
Twitter – https:/
LinkedIn – https:/
Pinterest – https:/
Reddit – https:/
Oncotarget is published by Impact Journals, LLC please visit http://www.
Media Contact
@RYANJAMESJESSUP
[email protected]
Original Source
https:/
Related Journal Article
http://dx.