OHIO researchers published the first structural biology analysis of a section of the COVID-19 viral RNA called the stem-loop II motif.
Credit: Jennifer Hines lab
ATHENS, Ohio (Jan. 20, 2021) – While the world awaits broad distribution of COVID-19 vaccines, researchers at Ohio University just published highly significant and timely results in the search for another way to stop the virus — by disrupting its RNA and its ability to reproduce.
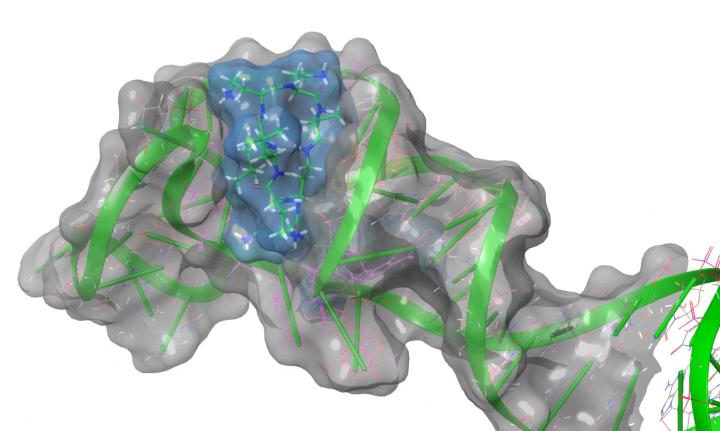
Dr. Jennifer Hines, a professor in the Department of Chemistry and Biochemistry, along with graduate and undergraduate students in her lab, published the first structural biology analysis of a section of the COVID-19 viral RNA called the stem-loop II motif. This is a non-coding section of the RNA, which means that it is not translated into a protein, but it is likely key to the virus’s replication.
“If you’ve been following the development of the vaccines, the current vaccines give our cells instructions to make a small piece of protein,” Hines said. “This protein fragment then triggers an immune response that will protect us if exposed to the real virus. In contrast, we are studying a section of RNA that does not code for proteins and is found in the coronavirus causing COVID-19 and other similar viruses. We are comparing it with the viral RNA from the SARS outbreak nearly 20 years ago — and looking for a possible target for an anti-viral drug that can attack the virus and stop it from reproducing. We are looking for the Achilles heel of the COVID-19 virus.
“The RNA genome of the coronavirus causing the COVID-19 pandemic is extremely efficient at commandeering human cells to produce more virus particles,” Hines added. “Our studies focused on a very small piece at the very end of this genome that is also present in other similar viruses. We found that the structural flexibility of this RNA motif may be related to its viral function and that it could potentially be disrupted with small molecules similar to the medicines we take to treat bacterial infections.”
Hines’ research found that the stem-loop II motif section of the viral RNA is “highly conserved in the COVID-19 virus, meaning that while other parts of the virus continue to evolve, this part of the virus is normally like a rock. And for the virus to cause disease, it needs to keep replicating inside the human body. So we are looking at this section of the RNA as a possible target for an antiviral.”
To learn more about this tiny section of COVID RNA, Hines’ lab took a big-data approach before starting the lab-based research. Using the Ohio Supercomputer Center, which provided priority, unbilled access to computational and storage resources for COVID-19 research, Hines found differences in the predicted three-dimensional RNA structure between the virus that caused the earlier SARS outbreak and the one causing the COVID-19 outbreak.
Shifting to Remote Courses and Research Led to COVID Discovery
When COVID-19 hit last spring, Hines was poised to have her medicinal chemistry class study anti-viral agents and her undergraduate tutorial students pursue computational RNA drug discovery.
“Despite and because of the COVID-19 pandemic, these classroom and tutorial topics have now turned into powerful online teachable moments,” Hines said at the time.
In class, her students used Blackboard’s Group Wiki tools to discuss molecular drug targets for inhibiting SARS-CoV-2, the virus that causes COVID-19. In her lab, students began to use the Ohio Supercomputer Center to investigate the three-dimensional structure of the RNA important for SARS-CoV-2 replication. Their work included docking drug molecules to the RNA to see if any existing drugs might potentially disrupt the RNA’s function.
“Three of the authors on the paper are Honors Tutorial College students, and two of these students — Hannah Boesger and Emily Marino — are the ones with whom the whole project started as part of their spring tutorial with me when we had to shift everything online for the second half of the semester,” Hines said.
“I personally view this as the most meaningful paper of my entire academic career partly due to the significance of the topic, but also due to the seamless combination of undergraduate and graduate teaching and research, which is what originally drew me to Ohio University and is what I believe is one of the University’s greatest strengths,” Hines said.
Finding a Potential Vulnerability
As their work progressed, the OHIO researchers, with graduate students Ali Aldhumani and Ismail Hossain in the lead, focused in on a single nucleotide, how it differs between the two outbreaks, and where it might make the RNA motif vulnerable to attack. Nucleotides are organic molecules that are the building blocks of nucleic acids, and thus of RNA and DNA.
“Using complementary biochemical and computational methods, we determined that the structural flexibility of this important noncoding RNA motif differs compared to that in the early 2000s SARS-CoV outbreak by only a single nucleotide change. We also identified FDA-approved drugs that bind the RNA motif and alter its flexibility. This was an exciting observation since the structure and flexibility of noncoding RNA affects its function, indicating that it may be possible to develop anti-viral therapeutics that specifically target this RNA motif and disrupt its function.”
###
The lead authors on the paper are graduate students Ali Aldhumani and Ismail Hossain, both pursuing a PhD in Chemistry at OHIO, along with graduate student Emily Fairchild, pursuing a masters in Chemistry at OHIO. In addition to Hannah Boesger and Emily Marino, the third undergraduate author is Mason Myers, recently named a Goldwater Scholar.
The Hines lab paper is titled “RNA Sequence and Ligand Binding Alter Conformational Profile of SARS-CoV-2 Stem Loop II Motif.” It is published in the journal Biochemical and Biophysical Research Communications.
Media Contact
Jim Sabin
[email protected]
Original Source
https:/




