Mutations associated with autism can inhibit the release of the bonding hormone oxytocin and cause abnormal social behavior in mice
Credit: Tokyo University of Science
Autism spectrum disorder is a neurodevelopmental condition involving impaired social abilities, and this makes it a fascinating subject for neuroscientists like Prof. Teiichi Furuichi of the Tokyo University of Science who study the neuroscience of social behavior. Prof. Furuichi and his colleagues have previously worked on developing mouse models of autism to unravel the condition’s neurochemical mechanisms, and in a paper recently published in the prestigious Journal of Neuroscience, they provide evidence that a genetic mutation associated with autism can impair the release of a peptide called oxytocin that plays an important role in regulating social behavior. This finding promises to broaden our understanding of the neurobiology of social behavior.
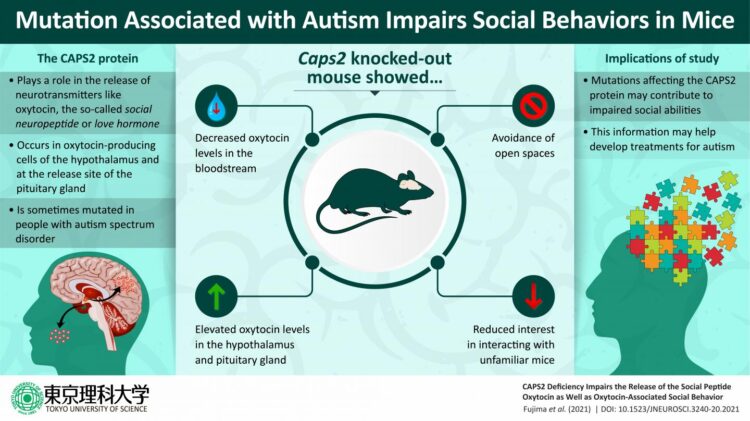
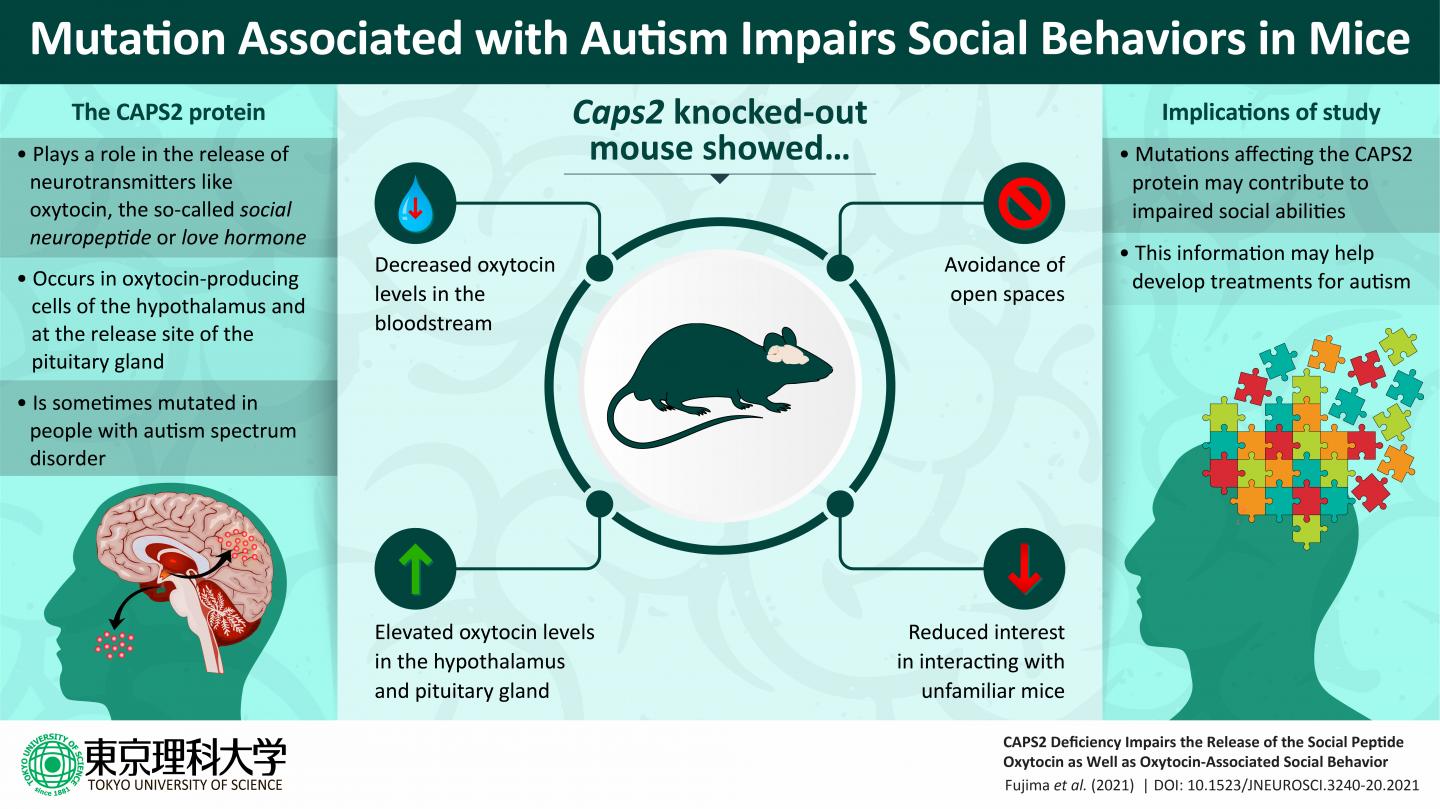
The gene that Prof. Furuichi’s team chose to study is Caps2, which encodes a protein called Ca2+-dependent activator protein for secretion 2 (CAPS2) that regulates the release of brain chemicals (or “neurotransmitters”). Previous studies have shown that CAPS2 deficiencies in mice cause behavioral impairments such as reduced sociality, increased anxiety, and disrupted circadian rhythms. Furthermore, a study of Japanese patients with autism spectrum disorder revealed that some of them had Caps2 mutations that adversely affect the CAPS2 protein’s functions. Prof. Furuichi and his colleagues had previously discovered that the CAPS2 protein is expressed in neurons in the hypothalamus and pituitary gland that release the neuropeptide oxytocin. This information formed the basis of their recent study. As Prof. Furuichi explains, “We hypothesized that CAPS2 deficiencies in mice should alter oxytocin release, which should in turn result in impaired social behavior.”
To test this hypothesis, researchers Shuhei Fujima, Graduate Student at Tokyo University of Science; Yoshitake Sano, Junior Associate Professor at Tokyo University of Science; Yo Shinoda, Associate Professor at Tokyo University of Pharmacy and Life Sciences; Tetsushi Sadakata, Associate Professor in Gunma University; Manabu Abe, Associate Professor at Niigata University; and Kenji Sakimura, a Fellow of Niigata University, among others, led by Prof. Furuichi conducted a series of experiments involving mice that carried genetic alterations that prevented them from expressing the CAPS2 protein. These mice had lower-than-normal oxytocin levels in their blood but higher-than-normal oxytocin levels in the hypothalamus and pituitary gland. The researchers interpreted this finding as evidence that CAPS2 deficiencies impede the normal release of oxytocin from these brain regions into the bloodstream.
Unsurprisingly, the reduced bloodstream levels of oxytocin had clear behavioral effects. When placed inside a rectangular box, the oxytocin neuron-specific CAPS2-deficient mice were unwilling to spend much time in the center of the box, and the researchers interpreted this as evidence of increased anxiety about the risk of a predator attacking them. The CAPS2-deficient mice also exhibited diminished willingness to engage in social interactions when introduced to unfamiliar mice. Interestingly, spraying an oxytocin solution into the noses of the CAPS2-deficient mice acted to restore their willingness to socially interact with unfamiliar mice.
Based on these findings, Prof. Furuichi and his colleagues conclude that the CAPS2 protein plays a critical role in facilitating the release of peripheral oxytocin into the bloodstream. They similarly suggest that CAPS2 is also involved in the release of central oxytocin into the brain regions relating to the control of sociality. Given the key role that oxytocin plays in regulating social behaviors, this could help to explain how mutations in the Caps2 gene could lead to atypical patterns of social behavior in persons with autism spectrum disorder. When asked about the social significance of his team’s work, Prof. Furuichi remarks, “We believe that this research, although basic, is an important achievement that will contribute to the development of tools for the early molecular diagnosis and effective treatment of autism spectrum disorder.”
Given the relatively high prevalence of autism and how extremely disabling severe cases can be, the development of effective treatments would have major benefits for people with autism and the society as a whole.
###
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society”, TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https:/
About Professor Teiichi Furuichi from Tokyo University of Science
Teiichi Furuichi, PhD, has served as a Professor at the Tokyo University of Science since 2011. He has previously worked at the RIKEN Brain Science Institute, Saitama University, Hiroshima University, the University of Tokyo, Japan’s National Institute for Basic Biology, and the State University of New York at Stony Brook. His research interests include the molecular mechanisms of brain development and the transcriptomic basis of postnatal development of the mouse cerebellum. He has authored over 150 original articles.
Funding information
This work was supported by the Japan Society for the Promotion of Science; the Japanese Ministry of Education, Culture, Sports, Science and Technology; and the NOVARTIS Foundation (Japan) for the Promotion of Science.
Media Contact
Tsutomu Shimizu
[email protected]
Original Source
https:/
Related Journal Article
http://dx.