Macrophages in fatty breast tissue promote triple-negative breast cancers
Credit: Becker, et al.
Smoking has long been the biggest cause of cancer in the United States, but obesity, now the second leading cause, has been gaining ground. A new study from researchers at the University of Chicago finds that women with breast cancer, the most common cancer among women, are at even higher risk from obesity.
Breast cancers occur in adipose tissue, better known as fat. Triple-negative breast cancer (TNBC) is a type of breast cancer that is particularly difficult to treat. None of the three most appealing drug targets — the estrogen receptor, the progesterone receptor and human epidermal growth factor receptor 2 — are present on TNBC cells.
“These cancers can be particularly aggressive,” said study author Lev Becker, PhD, an assistant professor in the Ben May Department for Cancer Research at the University of Chicago. “For patients with TNBC, there are few therapeutic options. The survival rate is quite low. And the cancer tends to be dramatically elevated in patients who are overweight or obese.”
Obesity has become “a global epidemic,” Becker said. The prevalence in the United States is about 36 percent for ages 20 to 39, 43 percent for ages 40 to 59, and 41 percent for those 60 and older. The United States is ranked 12th worldwide for obesity.
“Current treatment of breast cancer patients ignores the ongoing obesity epidemic,” said study co-author Marsha Rosner, PhD, the Charles B. Huggins Professor in the Ben May Department for Cancer Research. “In order to take this into consideration, we need to help patients lose weight or identify new drug targets that would be effective in obese cancer patients.”
Unfortunately, once the cancer has been detected there may not be time to lose weight prior to treatment. “So our bottom line,” Rosner said, is to “promote weight loss as a cancer prevention measure, incorporate weight loss as a component of therapy for patients with breast cancer, and develop specific drug targets that could be leveraged to address the obesity component of the disease.”
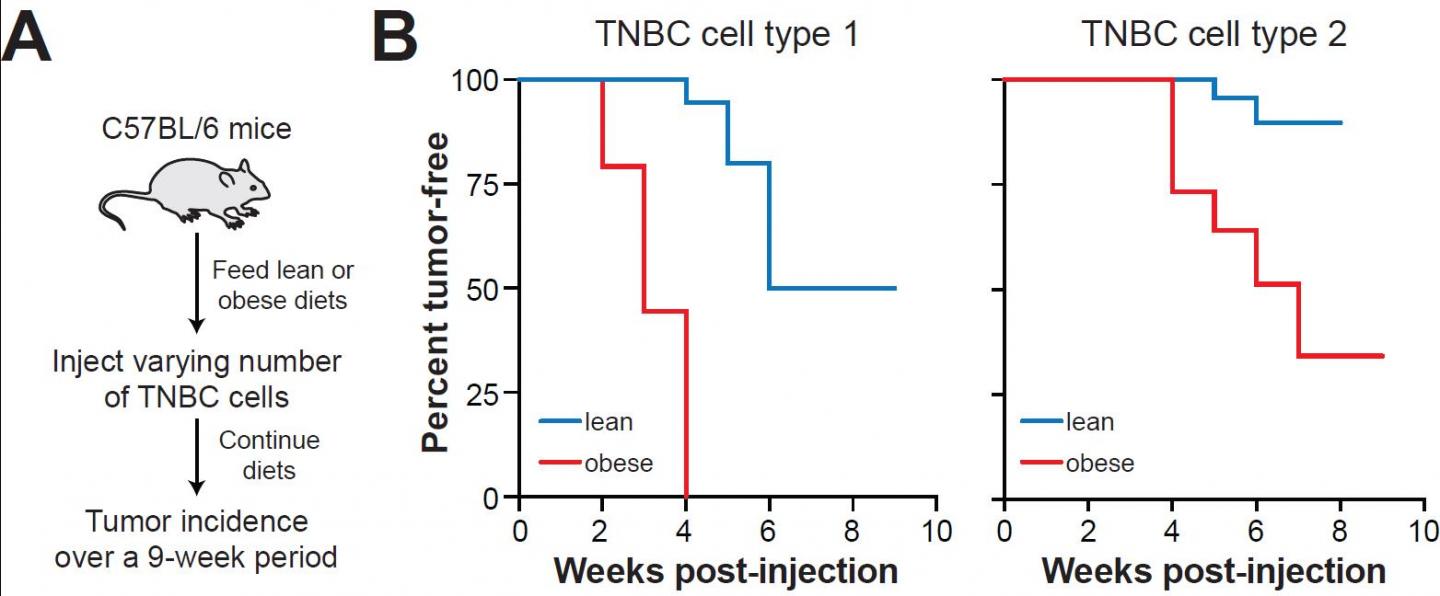
In their paper, “Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer,” published May 3, 2019 in the Journal of Experimental Medicine, Becker, Rosner and colleagues unravel the biology of how obesity promotes TNBC. They show that obesity reprograms macrophages — scavenger white blood cells that can devour invaders such as bacteria, viruses or tumor cells — into pro-inflammatory, metabolically-activated macrophages. Instead of fighting breast cancer, these immune cells actually promote it.
“Our studies, in mice and humans,” Becker added, “implicate these metabolically-activated adipose tissue macrophages.” They accumulate in mammary adipose tissue. They release interleukin 6, a pro-inflammatory cytokine which can fuel tumorigenesis. And they thrive on obesity.
Interleukin 6 binds to a receptor on the surface of existing cancer cells. That can create “an even more aggressive stem cell phenotype,” Becker said. “These cancer stem cells are able to encourage tumor growth and metastasis, enabling them to travel to other sites.” Patients with advanced or metastatic cancer have higher levels of IL-6 in their blood, which is correlated with poor survival rates.
Obesity, the study authors wrote, is a pathological state that “facilitates tumorigenesis by creating tumor permissive conditions in multiple tissues.” This suggests that chronic inflammation and its effects on tumorigenesis may be reversed by targeted anti-inflammatory therapies or by weight loss. Indeed, the researchers found that inducing weight loss in obese mice by feeding them a healthier low-fat diet reversed macrophage inflammation and TNBC tumor formation in mammary fat, even though their body weight remained elevated. These findings highlight the potential value of weight loss, not only as a preventive intervention but even after patients develop breast cancer.
###
This research was supported by grants from the National Institutes of Health, the Cancer Research Foundation, the Avon Foundation, the Bernice Goldblatt Endowment Fellowship, the University of Chicago, and the Department of Health via a National Institute for Health Research Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust and King’s College, London.
Additional authors of the study were Payal Tiwari, Ariane Blank, Chang Cui, Kelly Schoenfelt, Guolin Zhou, Galina Khramtsova and Funmi Olopade from the University of Chicago Medicine; Ajay M. Shah from King’s College, London; and Yanfei Xu and Seema A. Khan from Northwestern University.
Media Contact
John Easton
[email protected]
Related Journal Article
http://dx.




