Credit: Kai Yang
In a report published in NANO, scientists from the Jiangxi University of Science and Technology, Guangdong University of Petrochemical technology, Gannan Medical University and Nanchang Hangkong University in China underline the importance of defect engineering to promote catalytic performance by providing a simple and efficient way for modifying and optimizing metal-free semiconductor photocatalyst graphitic carbon nitride (g-C3N4) to solve the dual problems of environmental pollution and lack of fossil resources.
With the rapid growth of industrialization and population, environmental pollution and shortage of fossil resources have become two major challenges for sustainable social development in the 21st century. Hence, the development of green treatment technology is an imperative.
Semiconductor photocatalysis technology has become one of the most promising strategies due to its green, nontoxic and high efficiency by using solar energy. Recently, graphitic carbon nitride (g-C3N4), as a new semiconductor photocatalyst of nonmetallic polymer, has attracted wide attention in photo-catalytic field due to its better stability and optical properties. The photocatalytic activity of bare g-C3N4 is unsatisfactory due to its smaller surface area and rapid recombination of photogenerated carriers under visible light irradiation.
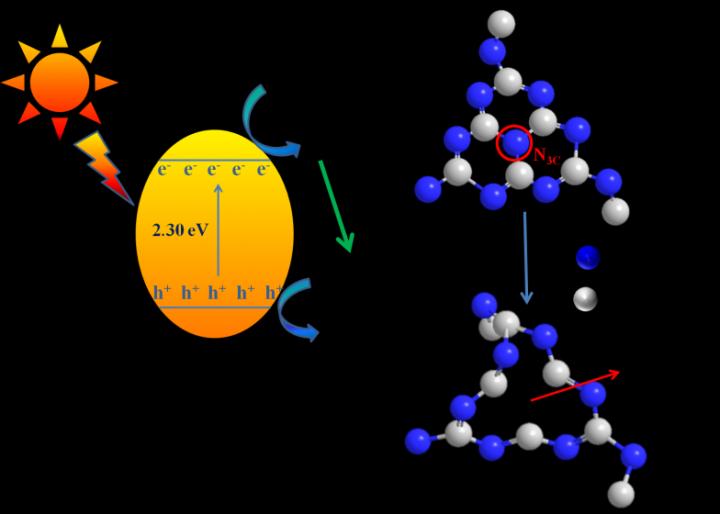
In this work, urea was used as the activated support for the nitrogen vacancies on the basis of bare g-C3N4 by the calcination of melamine. This led to great improvement of photocatalytic performance for the degradation of organic dyes in water, such as rhodamine (RhB), acid orange II, methyl orange (MO) and methyl blue (MB) under visible light irradiation (λ > 420nm). Thus, electrocatalytic performance for hydrogen evolution was achieved due to broader light response, efficient generation and migration of electron/hole charge carriers.
It is hoped that this research will provide an idea about the innovative design, synthesis and fabrication of modifying g-C3N4 and other N-based photocatalysts. There is potential for applying this catalyst to the treatment of environmental pollutants and the preparation of new energy.
###
This work was financially supported by the National Natural Science Foundation of China (Nos. 21962006, 21707055, 21607064 and 21567008), Youth Key Project of Jiangxi Province Nature Science Foundation (20192ACBL21011), Program of Qingjiang Excellent Young Talents of Jiangxi University of Science and Technology (No. 3401223429), Program of 5511 Talents in Scientific and Technological Innovation of Jiangxi Province (No. 20165BCB18014), Academic and Technical Leaders of the Main Disciplines in Jiangxi Province (No. 20172BCB22018), Jiangxi Province Natural Science Foundation (Nos. 20181BAB213010 and 20181BAB203018), Project Supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), Young Science Foundation of Jiangxi Province Education Office (No. GJJ160671), Open Project Program of the State Key Laboratory of Photocatalysis on Energy and Environment in Fuzhou University (No. SKLPEE-KF201712) and Open Fund of Guangdong Provincial Key Laboratory of Petrochemical Pollution Process and Control, Guangdong University of Petrochemical Technology (No. 2018B030322017).
Additional co-authors of the NANO paper are Kailian Zhang from Jiangxi University of Science and Technology, Man Zhou from Gannan Medical University, Shi Yang from Jiangxi University of Science and Technology, Xiaoshuai Ma from Jiangxi University of Science and Technology, Changlin Yu from Guangdong University of Petrochemical Technology, Yu Xie from Nanchang Hangkong University, Weiya Huang from Jiangxi University of Science and Technology and Qizhe Fan from Guangdong University of Petrochemical Technology.
Corresponding author for this study is Kai Yang, [email protected].
For more insight into the research described, readers are invited to access the paper on NANO.
IMAGE
Caption: A schematic illustration of modified g-C3N4 by nitrogen vacancies and reaction mechanism. The nitrogen vacancies not only increased the specific surface area, exposed more active sites, but also inhibited the recombination of photogenerated carriers on the surface of photocatalysts, resulting in the enhancement of photocatalytic and electrocatalytic performances.
Please contact Kai Yang ([email protected]) for usage restrictions on the picture.
NANO is an international peer-reviewed monthly journal for nanoscience and nanotechnology that presents forefront fundamental research and new emerging topics. It features timely scientific reports of new results and technical breakthroughs and publishes interesting review articles about recent hot issues.
Media Contact
Tay Yu Shan
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




