Credit: K. Dill/NIST
In a technique known as DNA origami, researchers fold long strands of DNA over and over again to construct a variety of tiny 3D structures, including miniature biosensors and drug-delivery containers. Pioneered at the California Institute of Technology in 2006, DNA origami has attracted hundreds of new researchers over the past decade, eager to build receptacles and sensors that could detect and treat disease in the human body, assess the environmental impact of pollutants, and assist in a host of other biological applications.
Although the principles of DNA origami are straightforward, the technique’s tools and methods for designing new structures are not always easy to grasp and have not been well documented. In addition, scientists new to the method have had no single reference they could turn to for the most efficient way of building DNA structures and how to avoid pitfalls that could waste months or even years of research.
That’s why Jacob Majikes and Alex Liddle, researchers at the National Institute of Standards and Technology (NIST) who have studied DNA origami for years, have compiled the first detailed tutorial on the technique. Their comprehensive report provides a step-by-step guide to designing DNA origami nanostructures, using state-of-the-art tools. Majikes and Liddle described their work in the Jan .8 issue of the Journal of Research of the National Institute of Standards and Technology.
“We wanted to take all the tools that people have developed and put them all in one place, and to explain things that you can’t say in a traditional journal article,” said Majikes. “Review papers might tell you everything that everyone’s done, but they don’t tell you how the people did it. ”
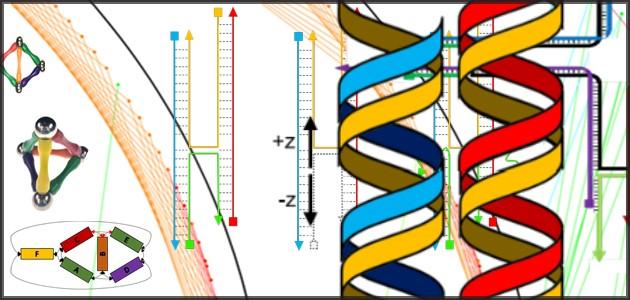
DNA origami relies on the ability of complementary base pairs of the DNA molecule to bind to each other. Among DNA’s four bases — adenine (A), cytosine (C), guanine (G) and thymine (T) — A binds with T and G with C. This means that a specific sequence of As, Ts, Cs and Gs will find and bind to its complement.
The binding enables short strands of DNA to act as “staples,” keeping sections of long strands folded or joining separate strands. A typical origami design may require 250 staples. In this way, the DNA can self-assemble into a variety of shapes, forming a nanoscale framework to which an assortment of nanoparticles — many useful in medical treatment, biological research and environmental monitoring — can attach.
The challenges in using DNA origami are twofold, said Majikes. First, researchers are fabricating 3D structures using a foreign language — the base pairs A, G, T and C. In addition, they’re using those base-pair staples to twist and untwist the familiar double helix of DNA molecules so that the strands bend into specific shapes. That can be difficult to design and visualize. Majikes and Liddle urge researchers to strengthen their design intuition by building 3D mock-ups, such as sculptures made with bar magnets, before they start fabrication. These models, which can reveal which aspects of the folding process are critical and which ones are less important, should then be “flattened” into 2D to be compatible with computer-aided design tools for DNA origami, which typically use two-dimensional representations.
DNA folding can be accomplished in a variety of ways, some less efficient than others, noted Majikes. Some strategies, in fact, may be doomed to failure.
“Pointing out things like ‘You could do this, but it’s not a good idea’ — that type of perspective isn’t in a traditional journal article, but because NIST is focused on driving the state of technology in the nation, we’re able to publish this work in the NIST journal,” Majikes said. “I don’t think there’s anywhere else that would have given us the leeway and the time and the person hours to put all this together.”
Liddle and Majikes plan to follow up their work with several additional manuscripts detailing how to successfully fabricate nanoscale devices with DNA.
###
Media Contact
Ben P. Stein
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




