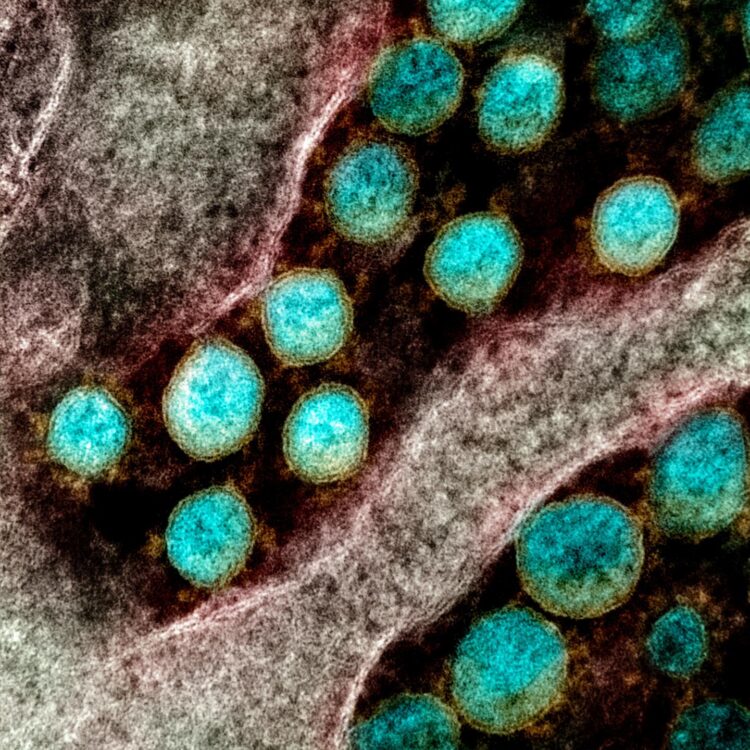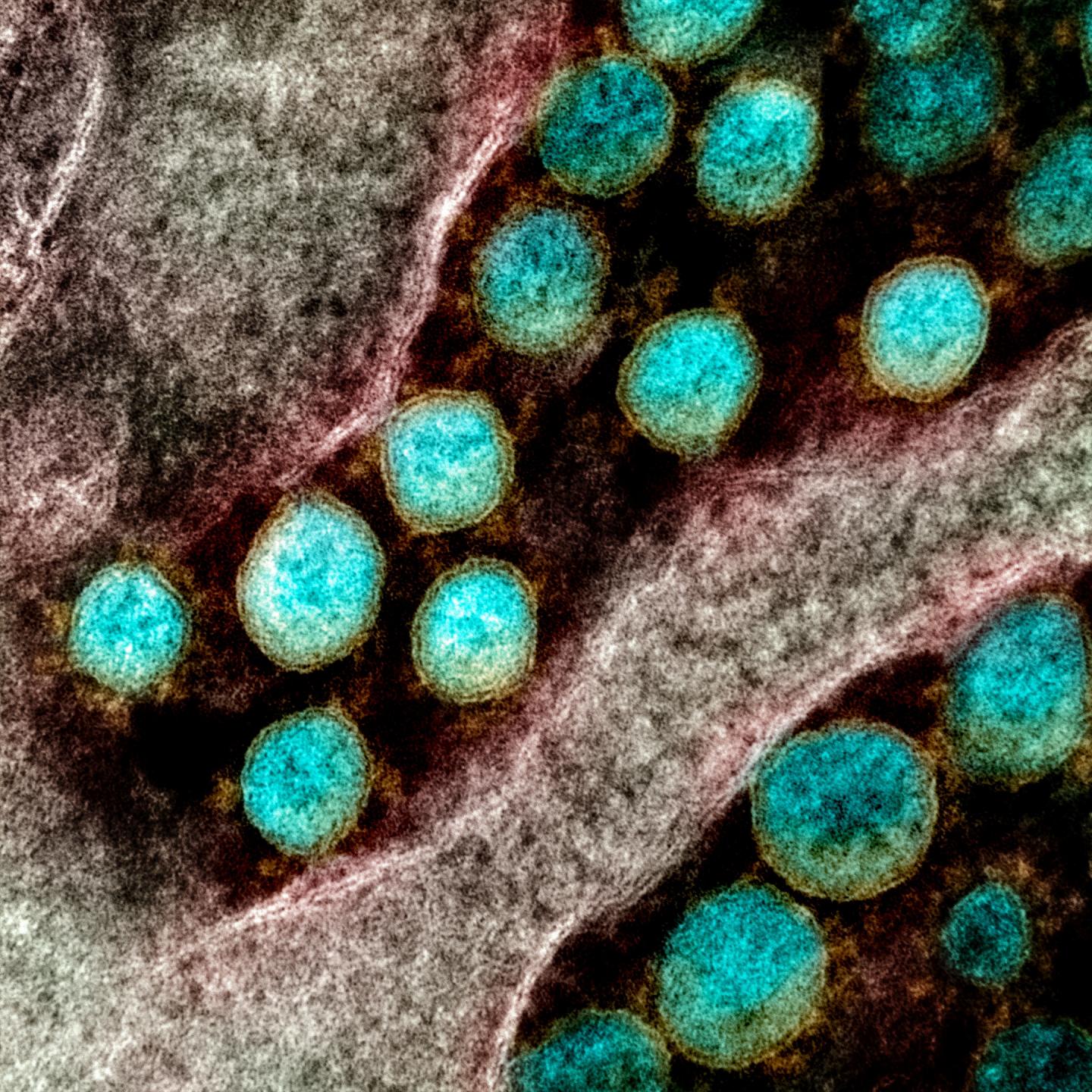
Credit: NIAID
WHAT:
A harmonized and collaborative approach to the clinical testing, scale-up and distribution of candidate vaccines to prevent COVID-19 is essential, scientific leaders write in a perspective published today in Science. As the COVID-19 pandemic continues, government, industry and academia have introduced a variety of vaccine candidates. The authors note that more than one effective vaccine approach likely will be required to successfully protect the global community from SARS-CoV-2, the virus that causes COVID-19. They describe a strategic approach to research and development that would generate essential data for multiple vaccine candidates in parallel.
National Institutes of Health Director Francis S. Collins, M.D., Ph.D., National Institute of Allergy and Infectious Diseases (NIAID) Director Anthony S. Fauci, M.D., Lawrence Corey, M.D., professor in the Vaccine and Infectious Disease Division at the Fred Hutchinson Cancer Research Center in Seattle, and John R. Mascola, M.D., director of NIAID’s Vaccine Research Center are the co-authors of the commentary.
The perspective discusses diverse vaccine candidates and key considerations for development, including the characteristics of various vaccine platforms in terms of prior commercial experience, scalability, and the types of immune responses generated. It also emphasizes that no single vaccine or vaccine platform is likely to meet the global need, highlighting the need for a coordinated strategic approach to vaccine development.
The authors stress that researchers need to learn more about what constitutes a durable protective immune response against COVID-19. They review considerations for vaccine efficacy trials, explaining how trials for several candidate vaccines can be conducted in parallel to generate essential safety and efficacy data and accelerate the licensure and distribution of COVID-19 vaccines. The authors propose specific approaches to harmonizing the clinical testing of multiple vaccine products, including using common clinical trials designs, clinical endpoints, standardized immune assays and a common Data Safety and Monitoring Board.
The authors emphasize that developing COVID-19 vaccines will require unprecedented cooperation from governments, academic institutions, industry, and global philanthropic partners. The ACTIV (Accelerating COVID-19 Therapeutic Interventions and Vaccines) public-private partnership spearheaded by NIH aims to facilitate such collaboration with discussions and collaborations on trial designs and data sharing.
Protecting the entire global community from COVID-19 through vaccination will require significant manufacturing capacity, according to the authors. They emphasize the need to fund the necessary biomanufacturing infrastructure and note possible hurdles in the eventual delivery of vaccines, including cost, distribution systems and cold chain requirements. The authors conclude that strategic collaboration among public and private sectors to effectively accelerate COVID-19 vaccine development is essential.
###
ARTICLE:
Corey et al. A Strategic Approach to COVID-19 Vaccine R&D. Science DOI: 10.1126/science.abc5312 (2020).
WHO:
NIH Director Francis S. Collins, M.D., Ph.D., NIAID Director Anthony S. Fauci, M.D., and NIAID Vaccine Research Center Director John Mascola, M.D., are available to provide comment.
CONTACT:
To schedule interviews with Dr. Fauci or Dr. Mascola, please contact Elizabeth Deatrick, (301) 402-1663, [email protected].
To schedule interviews with Dr. Collins, please contact the NIH News Media Branch, (301) 496-5787, [email protected]
NIAID conducts and supports research–at NIH, throughout the United States, and worldwide–to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.
NIH…Turning Discovery Into Health®
Media Contact
Elizabeth Deatrick
[email protected]
Original Source
https:/
Related Journal Article
http://dx.