Study demonstrates that RNA terminated by Hepatitis C Drug Sofosbuvir is more resistant to SARS-CoV-2 proofreader than RNA terminated by Remdesivir, and supports the use of Sofosbuvir with other antivirals for COVID-19 clinical trials
Credit: Jingyue Ju/Columbia Engineering
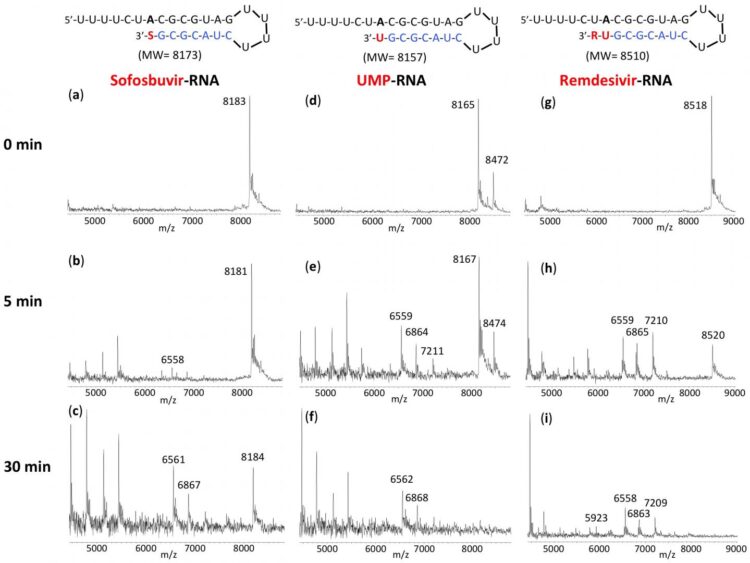
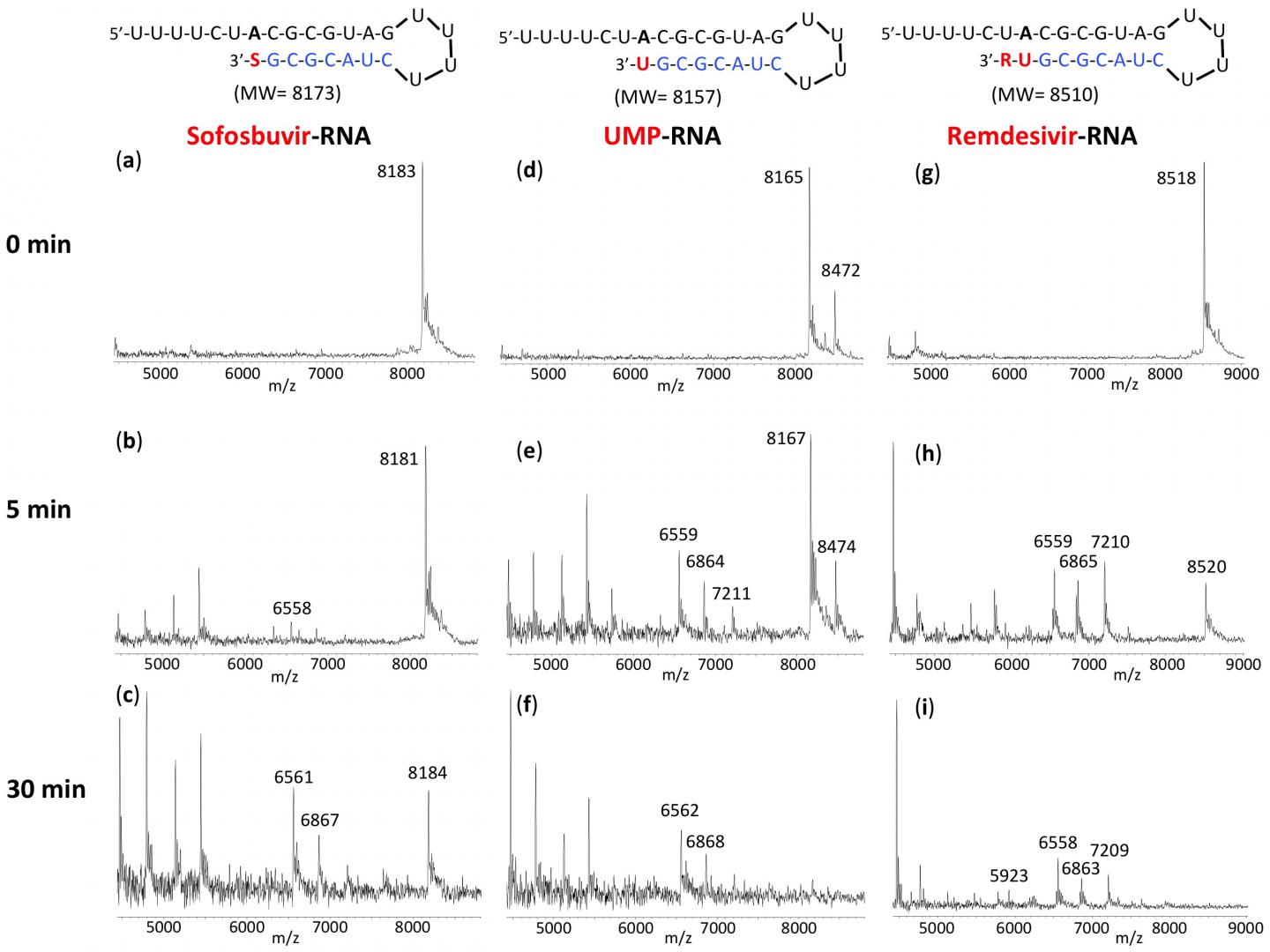
New York, NY–October 6, 2020–Columbia Engineering researchers report that Sofosbuvir-terminated RNA is more resistant to the proofreader of SARS-CoV-2, the virus that causes COVID-19, than Remdesivir-terminated RNA. The results of the new study, published today by the Nature Research journal Scientific Reports, support the use of the FDA-approved hepatitis C drug EPCLUSA–Sofosbuvir/Velpatasvir–in combination with other drugs in COVID-19 clinical trials.
The SARS-CoV-2 exonuclease-based proofreader maintains the accuracy of viral RNA genome replication to sustain virulence. Any effective antiviral targeting the SARS-CoV-2 polymerase must therefore display a certain level of resistance to this proofreading activity.
“We found that the RNA terminated by Sofosbuvir resists removal by the exonuclease to a substantially higher extent than RNA terminated by Remdesivir, another drug being used as a COVID-19 therapeutic,” says the team’s lead PI Jingyue Ju, Samuel Ruben-Peter G. Viele Professor of Engineering; professor of Chemical Engineering and Pharmacology; director, Center for Genome Technology & Biomolecular Engineering.
The new study builds upon earlier work the researchers have conducted. Last January, before COVID-19 reached pandemic status, the team posited that EPCLUSA might inhibit SARS-CoV-2, the virus responsible for COVID-19. Their reasoning was based on the analysis of the molecular structures and activities of hepatitis C viral inhibitors and a comparison of hepatitis C virus and coronavirus replication.
In a subsequent study, the researchers demonstrated that the active drug Sofosbuvir triphosphate is incorporated by SARS-CoV and SARS-CoV-2 polymerases, shutting down the polymerase reaction. Other investigators have since demonstrated the ability of Sofosbuvir to inhibit SARS-CoV-2 replication in lung and brain cells; currently, COVID-19 clinical trials with a number of hepatitis C drugs such as EPCLUSA and the combination of Sofosbuvir and Daclatasvir (which is similar to Velpatasvir) are ongoing in several countries.
Ju notes that a recent preprint from UC Berkeley indicates that a combination of Remdesivir and EPCLUSA increases Remdesivir’s efficacy 25-fold in inhibiting SARS-CoV-2, the virus that causes COVID-19: “These results offer a molecular basis supporting the study of EPCLUSA in combination with Remdesivir for COVID-19 clinical trials.”
###
About the Study
The study is titled “Sofosbuvir terminated RNA is more resistant to SARS CoV 2 proofreader than RNA terminated by Remdesivir.”
Authors are: Steffen Jockusch1,4,5, Chuanjuan Tao1,2,5, Xiaoxu Li1,2, Minchen Chien1,2, Shiv Kumar1,2, Irina Morozova1,2, Sergey Kalachikov1,2, James J. Russo1,2 & Jingyue Ju1,2,3
1Center for Genome Technology and Biomolecular Engineering, Columbia University
2Department of Chemical Engineering, Columbia Engineering
3Department of Molecular Pharmacology and Therapeutics, Columbia University
4Department of Chemistry, Columbia University
The study was supported by Columbia University, a grant from the Jack Ma Foundation, a generous gift from the Columbia Engineering Member of the Board of Visitors Dr. Bing Zhao, and Fast Grants.
The authors declare no competing interests.
LINKS:
Paper: http://www.
DOI: 10.1038/s41598-020-73641-9
http://engineering.
https:/
https:/
https:/
https:/
https:/
Columbia Engineering
Columbia Engineering, based in New York City, is one of the top engineering schools in the U.S. and one of the oldest in the nation. Also known as The Fu Foundation School of Engineering and Applied Science, the School expands knowledge and advances technology through the pioneering research of its more than 220 faculty, while educating undergraduate and graduate students in a collaborative environment to become leaders informed by a firm foundation in engineering. The School’s faculty are at the center of the University’s cross-disciplinary research, contributing to the Data Science Institute, Earth Institute, Zuckerman Mind Brain Behavior Institute, Precision Medicine Initiative, and the Columbia Nano Initiative. Guided by its strategic vision, “Columbia Engineering for Humanity,” the School aims to translate ideas into innovations that foster a sustainable, healthy, secure, connected, and creative humanity.
Media Contact
Holly Evarts
[email protected]
Original Source
https:/
Related Journal Article
http://dx.