Credit: Credit: Focke C, Blume T, Zott B, Shi Y, et al.
Researchers have discovered a way to better predict progression of Alzheimer’s disease. By imaging microglial activation levels with positron emission tomography (PET), researchers were able to better predict progression of the disease than with beta-amyloid PET imaging, according to a study published in the April issue of the Journal of Nuclear Medicine.
According to the Alzheimer’s Association, an estimated 5.3 million Americans are currently living with Alzheimer’s disease. By 2025, that number is expected to increase to more than seven million. The hallmark brain changes for those with Alzheimer’s disease include the accumulation of beta-amyloid plaques. When microglial cells from the central nervous system recognize the presence of beta-amyloid plaques, they produce an inflammatory reaction in the brain.
“The 18-kD translocator protein (TSPO) is highly expressed in activated microglia, which makes it a valuable biomarker to assess inflammation in the brain,” said Matthias Brendel, MD, MHBA, at Ludwig-Maximilians-University of Munich in Germany. “In our study, we utilized TSPO-PET imaging to determine whether microglial activation had any influence on cognitive outcomes in an amyloid mouse model.”
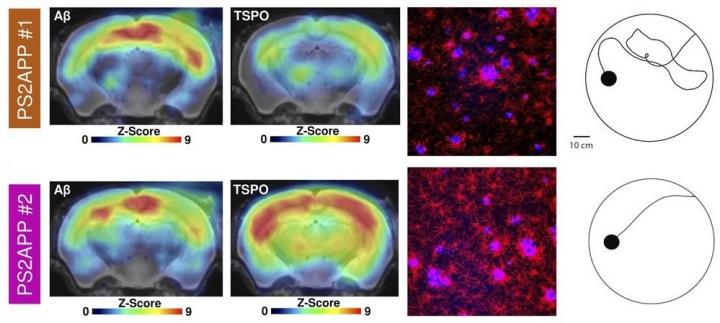
In the study, researchers compiled a series of PET images for 10 transgenic mice with beta-amyloid proteins and seven wild-type mice. TSPO PET imaging of activated microglia was conducted at eight, 9.5, 11.5 and 13 months, and beta-amyloid PET imaging was performed at eight and 13 months. Upon completion of the imaging, researchers then subjected the mice to a water maze in which the mice were to distinguish between a floating platform that would hold their weight and one that would sink. The tasks were performed several times a day during a 1.5-week period. Memory performance in the water maze was assessed by measuring the average travel time from the start point to a platform each day of training and by calculating the traveled distance at the last day of training. After completing the water maze task, immunohistochemistry analyses were performed for microglia, amyloid and synaptic density.
Transgenic mice with the highest TSPO PET signal in the forebrain or other areas associated with spatial learning tended to have better cognitive performance in the water maze, while beta-amyloid signals in the same areas of the brain showed no correlation to cognitive outcomes in the maze. Researchers found that an earlier microglial response to amyloid pathology in transgenic mice also protected synaptic density at follow-up. Specifically, transgenic mice with higher TSPO expression at eight months had much better cognitive outcomes in the water maze and higher synaptic density as confirmed by immunochemistry analyses.
“This study provides the first evidence that the level of microglial activation could be a far better predictor of current and future cognitive performance than beta-amyloid levels,” noted Brendel. “Keeping the limitations of mouse models in mind, it could be crucial to modify an individual’s microglial activation state to ameliorate future cognitive decline. We believe that a balanced microglia activation is crucial for prevention of cognitive impairment.”
###
The authors of “Early and Longitudinal Microglial Activation but Not Amyloid Accumulation Predicts Cognitive Outcome in PS2APP Mice” include Carola Focke, Maximilian Deussing, Claudio Schmidt, Simon Lindner, Franz-Josef Gildehaus, Leonie Beyer and Barbara von Ungern-Sternberg, Department of Nuclear Medicine, University Hospital of Munich, LMU Munich, Munich, Germany; Tanja Blume, Department of Nuclear Medicine, University Hospital of Munich, LMU Munich, Munich, Germany, and Center for Neuropathology and Prion Research, Ludwig-Maximilians-University of Munich, Munich, Germany; Benedikt Zott and Helmuth Adelsberger, Institute of Neuroscience, Technical University of Munich, Munich, Germany; Yuan Shi and Mario M. Dorostkar, Center for Neuropathology and Prion Research, Ludwig-Miximilians-University of Munich, Munich, Germany, and DZNE-German Center for Neurodegenerative Diseases, Munich, Germany; Finn Peters, DZNE-German Center for Neurodegenerative Diseases, Munich, Germany; Gernot Kleinberger, Munich Cluster for Systems Neurology, University of Munich, Munich, Germany, and Biomedical Center, Biochemistry, Ludwig-Maximilians-University of Munich, Munich, Germany; Peter Bartenstein and Matthias Brendel, Department of Nuclear Medicine, University Hospital of Munich, LMU Munich, Munich, Germany, and Munich Cluster for Systems Neurology, University of Munich, Munich, Germany; Laurence Ozmen and Karlheinz Baumann, Roche Pharma Research and Early Development, F. Hoffman-La Roche Ltd., Basel, Switzerland; Christian Haass, DZNE-German Center for Neurodegenerative Diseases, Munich, Germany, Munich Cluster for Systems Neurology, University of Munich, Munich, Germany, and Biomedical Center, Biochemistry, Ludwig-Maximilians-University of Munich, Munich, Germany; Jochen Herms, Center for Neuropathology and Prion Research, Ludwig-Maximilians-University of Munich, Munich, Germany, DZNE-German Center for Neurodegenerative Diseases, Munich, Germany, and Munich Cluster for Systems Neurology, University of Munich, Munich, Germany; Axel Rominger, Department of Nuclear Medicine, University Hospital of Munich, LMU Munich, Munich, Germany, Munich Cluster for Systems Neurology, University of Munich, Munich, Germany, and Department of Nuclear Medicine, Inselspital, University Hospital Bern, Bern, Switzerland.
This study was made available online in September 2018 ahead of final publication in print in April 2019.
For more information or to schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected]. Current and past issues of the Journal of Nuclear Medicine can be found online at http://jnm.
About the Society of Nuclear Medicine and Molecular Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging, vital elements of precision medicine that allow diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes.
SNMMI’s more than 17,000 members set the standard for molecular imaging and nuclear medicine practice by creating guidelines, sharing information through journals and meetings, and leading advocacy on key issues that affect molecular imaging and therapy research and practice. For more information, visit http://www.
Media Contact
Rebecca Maxey
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




