Credit: OIST
People with the same lifespan do not necessarily have the same quality of life. As we live longer, extending quality of life — “healthspan” — is gaining importance. Scientists at the Okinawa Institute of Science and Technology Graduate University (OIST) have discovered a gene linked with healthy ageing in the roundworm C. elegans, shedding light on the genetics of healthspan.
The team has identified a gene called elpc-2 in C. elegans that plays an important role in maintaining healthspan as the worm ages. This gene is conserved in humans — and worms with defects in this gene showed impaired movement as they aged. Movement at older ages is an indicator of healthspan in both humans and worms.
“As we age, some people keep full locomotor ability while others do not, and we want to understand the genetic reasons,” says Dr. Kazuto Kawamura, first author of the study, published in G3: Genes, Genomes, Genetics. “This gene is one among many playing a role in healthy ageing.”
“Our new experimental approach also allows us to test hundreds of worms simultaneously, which could be useful for other researchers.”
C. “elegance”
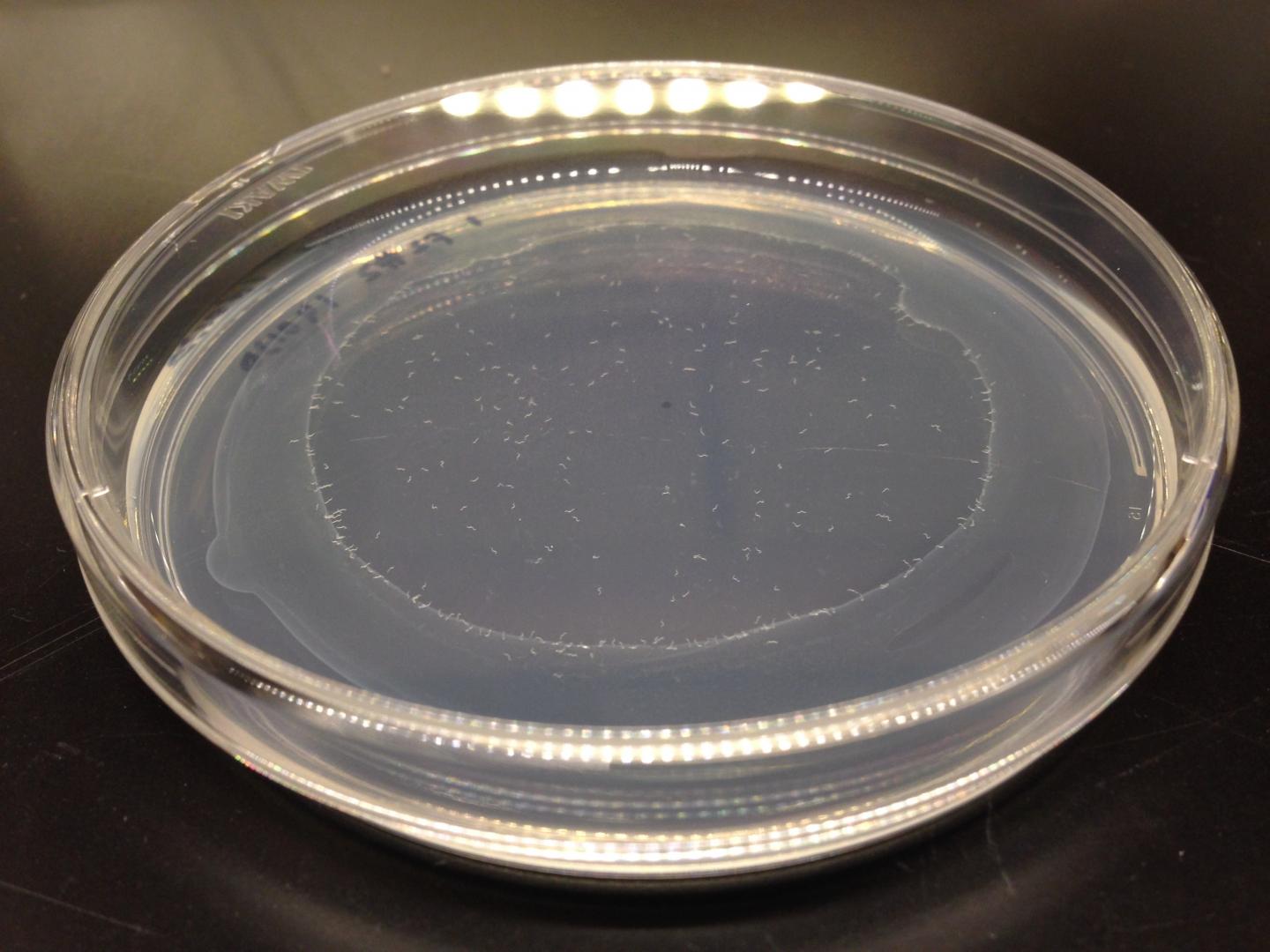
C. elegans is a useful model for studying ageing — it has a short lifespan and can be easily manipulated in the lab. Kawamura inserted random mutations into the genome of these worms. By studying the offspring of the mutated worms, he was able to test which mutations affected healthspan. He measured whether the organisms were able to maintain their ability to move toward a food source as they aged.
Worms were placed at the center of a dish with food at the edge. They naturally head towards food, providing that their movement is not impaired. Any worms that failed to reach the food on the first day were judged to have impaired movement in young age and were removed from the experiment — Kawamura was only interested in how this ability declined with age.
The remaining mutants were retested as they got older using the same approach, dubbed the “edge assay” because worms migrate to the edge to reach food. In this later testing, several worms showing impaired movement were identified.
These were then sequenced and their DNA was compared with a normal “wild type” worm to pinpoint the mutations and identify the genes responsible.
“Creating hundreds of random mutants is not so difficult,” says Kawamura, “but it is difficult to figure out which mutation is responsible for the impact on locomotor ability.”
Understanding healthspan
In this way, the researchers identified elpc-2 and its role in healthspan. The gene encodes part of the elongator complex, which has many important functions including orchestrating the correct folding of proteins. Some of these proteins, in turn, may have roles in locomotion.
Mutants with a damaged elpc-2 gene lacked a working elongator complex, which explains why movement was impaired. To confirm this, Kawamura injected these worms with a working copy of the gene, and movement was restored. He also created worms that expressed a fluorescent copy of the elongator complex, illustrating its widespread expression throughout the body.
Interestingly, other genes were identified that had a strong impact on healthspan — but not lifespan. In other words, the underlying mutations didn’t much affect how long a worm lived, but did impact on how they moved. This demonstrates that while healthspan and lifespan overlap, the genetic basis is distinct.
The elongator complex is just one part of the healthspan puzzle. Next, Kawamura intends to explore other genes playing a role in healthy ageing.
“Once we have a more complete picture of the genes involved, we can begin to manipulate them to improve healthspan,” says Kawamura. “First in C. elegans and perhaps, one day, humans.”
###
Media Contact
Tomomi Okubo
[email protected]
Related Journal Article
http://dx.




