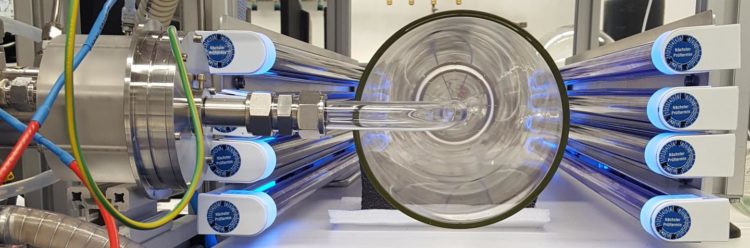
Laboratory results question current knowledge on the degradation of dimethyl sulfide within the sulfur cycle
Credit: Torsten Berndt, TROPOS
Leipzig. An international research team was able to experimentally show in the laboratory a completely new reaction path for the largest natural sulfur source in the atmosphere. The team from the Leibniz Institute for Tropospheric Research (TROPOS), the University of Innsbruck and the University of Oulu are now reporting in the Journal of Physical Chemistry Letters on the new degradation mechanism for dimethyl sulfide (DMS), which is released mainly by the oceans. The new findings show that important steps in the Earth’s sulfur cycle have not yet been properly understood, as they call into question the previously assumed formation pathways for sulfur dioxide (SO2), methanesulfonic acid (MSA) and carbonyl sulfide (OCS) based on DMS degradation, which strongly influence the Earth’s climate through the formation of natural particles and clouds.
In the laboratory studies, a free-jet flow system was used at TROPOS in Leipzig, which allows the investigation of oxidation reactions under atmospheric conditions without disturbing wall effects. The products of the reactions were measured with state-of-the-art mass spectrometers using different ionization methods. The investigations on the degradation process of dimethyl sulfide (DMS; CH3SCH3) showed that this predominantly proceeds by a two-step radical isomerization process, in which HOOCH2SCHO is formed as a stable intermediate product as well as hydroxyl radicals. There has been theoretical speculation about this reaction pathway for four years now, but the German-Austrian-Finnish team has only now been able to prove it. “The interaction of optimal reaction conditions and highly sensitive detection methods allows us to look almost directly into a reaction system,” reports Dr. Torsten Berndt from TROPOS, who is in charge of the investigations. The new reaction pathway is significantly faster than the traditional bimolecular radical reactions with nitrogen monoxide (NO), hydroperoxy (HO2) and peroxy radicals (RO2). “Further investigations on the degradation of the intermediate HOOCH2SCHO will hopefully give us clarity about the formation channels, especially of sulfur dioxide (SO2) and carbonyl sulfide (OCS),” Berndt continued about the upcoming investigations.
Dimethyl sulfide (DMS) is a sulfur-containing organic gas that occurs almost everywhere: the degradation product of bacteria, for example, is part of human bad breath. On the other hand, the large quantities of DMS that are produced and outgassed during decomposition processes in the ocean are important for the climate: Estimated 10 to 35 million metric tons from the seawater are released into the atmosphere every year. DMS is thus the largest natural source of sulfur for the atmosphere. As a result of its reaction with hydroxyl radicals, sulfuric acid (H2SO4) is formed starting from SO2 and methanesulfonic acid (MSA), which play a major role in the formation of natural particles (aerosols) and clouds over the oceans. Carbonyl sulfide (OCS) is also important, as its low reactivity in the atmosphere allows it to be mixed into the stratosphere, where it contributes to the formation of sulfuric acid aerosols and thus to the cooling of the Earth’s atmosphere.
The new findings about the degradation pathways of DMS help to improve the knowledge about the formation of natural aerosols. The contribution of aerosols and the resulting clouds is still the greatest uncertainty in climate models. In contrast to greenhouse gases such as carbon dioxide, cloud formation processes are much more complex and difficult to model.
###
Publication:
T. Berndt, W. Scholz, B. Mentler, L. Fischer, E. H. Hoffmann, A. Tilgner, N. Hyttinen, N. L. Prisle, A. Hansel, and H. Herrmann (2019): Fast Peroxy Radical Isomerization and OH Recycling in the Reaction of OH Radicals with Dimethyl Sulfide. J. Phys. Chem. Lett. 2019, 10, 21, 6478-6483. DOI: 10.1021/acs.jpclett.9b02567
https:/
The study was by the European Research Council (ERC), the European Union Framework Programme for Research and Innovation (Horizon-2020 project SURFACE (717022) and the MSCA programme (764991)) and the Academy of Finland (308238 & 31475).
Contacts:
Dr. Torsten Berndt
Scientific staff, Atmospheric Chemistry Department at the Leibniz Institute for Tropospheric Research (TROPOS), Leipzig, Germany
Phone +49-341-2717-7032
https:/
and
Prof. Hartmut Herrmann
Head of the Atmospheric Chemistry Department at the Leibniz-Institute for Tropospheric Research (TROPOS), Leipzig, Germany
Phone: +49-341-2717-7024
https:/
or
Tilo Arnhold
Public Relations at the Leibniz Institute for Tropospheric Research (TROPOS), Leipzig, Germany
Phone: +49-341-2717-7189
https:/
Links:
Blog in Nature Chemistry Community
https:/
Free-jet flow system at TROPOS:
https:/
Laboratory experiments on tropospheric multiphase processes at TROPOS:
https:/
Media Contact
Tilo Arnhold
[email protected]
49-341-271-77189
Original Source
https:/
Related Journal Article
http://dx.