Utilizing cutting-edge proteomics, researchers at the Buck Institute and elsewhere have mapped the “tau interactome” uncovering new findings about the role of tau in neurodegenerative disease. Publishing in Cell, scientists found that mutant tau impacts the function of mitochondria in human neurons. They also suggest a mechanism for how tau gets released from neurons and spreads throughout the brain, a pathological process that is strongly correlated with disease progression.
Credit: The Buck Institute for Research on Aging
Utilizing cutting-edge proteomics, researchers at the Buck Institute and elsewhere have mapped the “tau interactome” uncovering new findings about the role of tau in neurodegenerative disease. Publishing in Cell, scientists found that mutant tau impacts the function of mitochondria in human neurons. They also suggest a mechanism for how tau gets released from neurons and spreads throughout the brain, a pathological process that is strongly correlated with disease progression.
“Understanding the mechanisms of what is happening within cells during disease is key to discovering new ways to treat neurodegenerative diseases including Alzheimer’s, which is the most common tauopathy,” said Buck Institute assistant professor Tara Tracy, PhD, lead author of the paper. “We hope that other researchers take advantage of our ‘tau interactome’ which is a broad and unbiased survey of tau interacting proteins in the cell that could be contributing to disease.”
The properties of tau
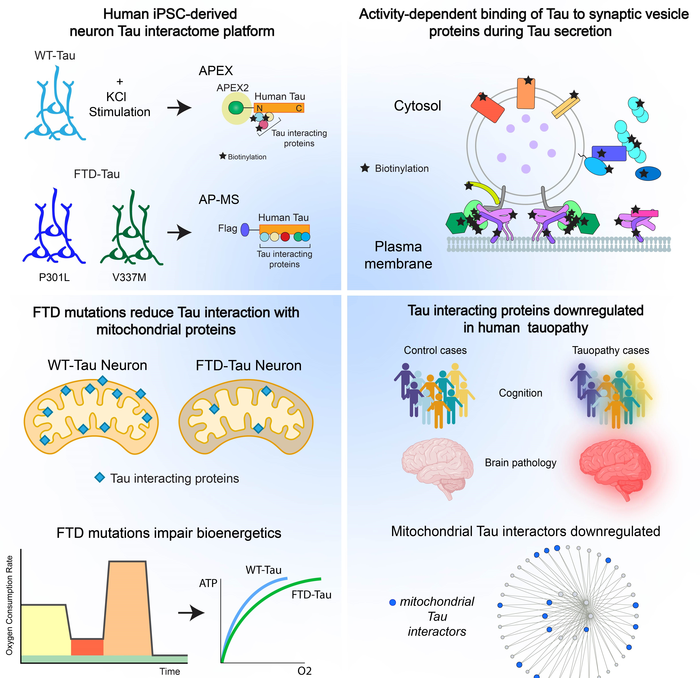
Normal tau is well-known for its role in binding to microtubules which maintain the cytoskeleton of the cell. In disease, abnormal chemical changes cause tau to detach from the microtubules and stick to other tau proteins forming threads that eventually join to become tangles inside of neurons. The presence of tau tangles are one of the hallmarks of Alzheimer’s disease and related tauopathies.
Tracy says over the last decade researchers realized that, in disease, tau is doing a lot more than just impacting the cytoskeleton of the cell. “Tau interactions are more complex than what was initially thought. There’s been a lot of attention in the field to the fact that tau can be secreted from neurons and spread across connected cells — but there hasn’t been an understanding of how this occurs and the cellular machinery involved,” she said. “The methods used in this paper provide an unprecedented dynamic map of the tau interactome to shed light on the interactions that occur during tau secretion and on tau’s role in neuronal function and disease.”
New insights
Working in neurons derived from human induced pluripotent stem cells, researchers show that when tau is secreted during increased neuronal activity it interacts with proteins on the outside, rather than inside, of synaptic vesicles. These vesicles store neurotransmitters which are released at the junction between neurons. Tracy said this is surprising when it comes to tau, adding that the release likely happens through an association with the SNARE complex, proteins that exist at the presynaptic terminal and that are necessary for the fusion vesicles with the plasma membrane to release neurotransmitters. “Showing a potential mechanism for how tau gets released can inform future studies into how we can prevent diseased tau from getting out of neurons and spreading throughout the brain,” said Tracy.
Researchers also show that tau binds to mitochondrial proteins in neurons. Tracy says the binding appears to be beneficial when tau is normal, but when diseased tau impairs neuronal bioenergetics it may be due to tau’s diminishing interaction with mitochondrial proteins. These tau interacting proteins in mitochondria were downregulated in brain tissue from multiple human cohorts and the downregulation correlated with disease severity.
Important for Alzheimer’s and much more
Tauopathies encompass several clinical-pathological entities including Alzheimer’s disease, progressive supranuclear palsy, Pick’s disease, chronic traumatic encephalopathy, frontotemporal dementia, corticobasal degeneration, and post-encephalitic parkinsonism. “Millions of people worldwide are currently living with the burden of tauopathy-associated neurological diseases,” said Tracy. “This provides an urgency to those of us working to develop treatments for these diseases. It is our hope that this paper helps move the field forward in a major way.”
Citation: Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration
DOI: 10.1016/j.cell.2021.12.041
Buck Institute staff scientist Grant Kauwe collaborated on the study. Other collaborators include Danielle L. Swaney, Michael E. Ward, Erica Stevenson, Ruth Huttenhain, Xu Chen, Maria Telpoukhovskaia, Sang-Won Min, Chao Wang, Peter Dongmin Sohn, Jordie Martin, Yungui Zhou, and Nevan J. Krogan, Gladstone Institutes, San Francisco, CA; Jesus Madero-Perez, Csaba Konrad, Maria Mercedes, Lauren Sweetland-Martin, Man Ying Wong, Wenjie Luo, Shiaoching Gong, Giovanni Manfredi, and Li Gan, Brain and Mind Research Institute, Weill Cornell Medicine, NY, NY; Michelle Moritz, University of California, San Francisco; Timothy S. Chang, Giovanni Coppola, Daniel H. Geschwind, Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles; John Q. Trojanowski, Virginia M. Y. Lee, Center for Neurodegenerative Disease Research, University of Pennsylvania School of Medicine, Philadelphia, PA; Sue-Ann Mok, University of Alberta, Edmonton, Canada
Acknowledgments: This work was supported by NIH grants U54NS1000717, R01AG054214, K01AG057862 and RN25NS065723, the Tau Consortium and JPB Foundation, and Fundación Ramón Areces. The results are based on data obtained from the AMP-AD Knowledge Portal (https://adknowledgeportal.synapse.org/). Dr. Levey (Emory University) provided BLSA data from brain tissue collected through the NIA’s BLSA, UPenn data from brain tissue collected through the University of Pennsylvania, Emory data from brain tissue collected through Emory Alzheimer’s Disease Research Center Brain Bank, and Banner data generated in part from samples collected through the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona supported by the NINDS (U24 NS072026), the NIA (P30 AG19610), the Arizona Department of Health Services (contract 211002), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
DECLARATION OF INTERESTS
L.G. is a founder of Aeton Therapeutics. N.J.K. received research support from Vir Biotechnology and F. Hoffmann-La Roche, has consulting agreements with the Icahn School of Medicine at Mount Sinai, New York, Maze Therapeutics and Interline Therapeutics, is a shareholder in Tenaya Therapeutics, Maze Therapeutics and Interline Therapeutics, and a financially compensated Scientific Advisory Board Member for GEn1E Lifesciences, Inc.
About the Buck Institute for Research on Aging
At the Buck, we aim to end the threat of age-related diseases for this and future generations. We bring together the most capable and passionate scientists from a broad range of disciplines to study mechanisms of aging and to identify therapeutics that slow down aging. Our goal is to increase human health span, or the healthy years of life. Located just north of San Francisco, we are globally recognized as the pioneer and leader in efforts to target aging, the number one risk factor for serious diseases including Alzheimer’s, Parkinson’s, cancer, macular degeneration, heart disease, and diabetes. The Buck wants to help people live better longer. Our success will ultimately change healthcare. Learn more at: https://buckinstitute.org
Journal
Cell
DOI
10.1016/j.cell.2021.12.041
Method of Research
Experimental study
Article Title
Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration
Article Publication Date
20-Jan-2022




