Credit: University of Adelaide
Researchers at the University of Adelaide have found new evidence about the positive role of androgens in breast cancer treatment with immediate implications for women with estrogen receptor-driven metastatic disease.
Published today in Nature Medicine, the international study conducted in collaboration with the Garvan Institute of Medical Research, looked at the role of androgens – commonly thought of as male sex hormones but also found at lower levels in women – as a potential treatment for estrogen receptor positive breast cancer.
Watch a video explainer about the new study at – https:/
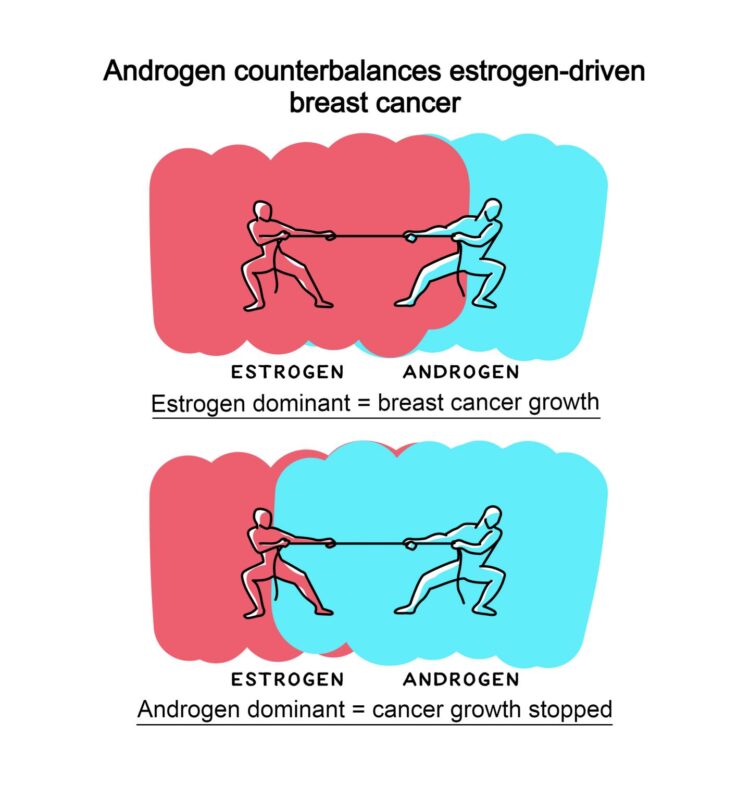
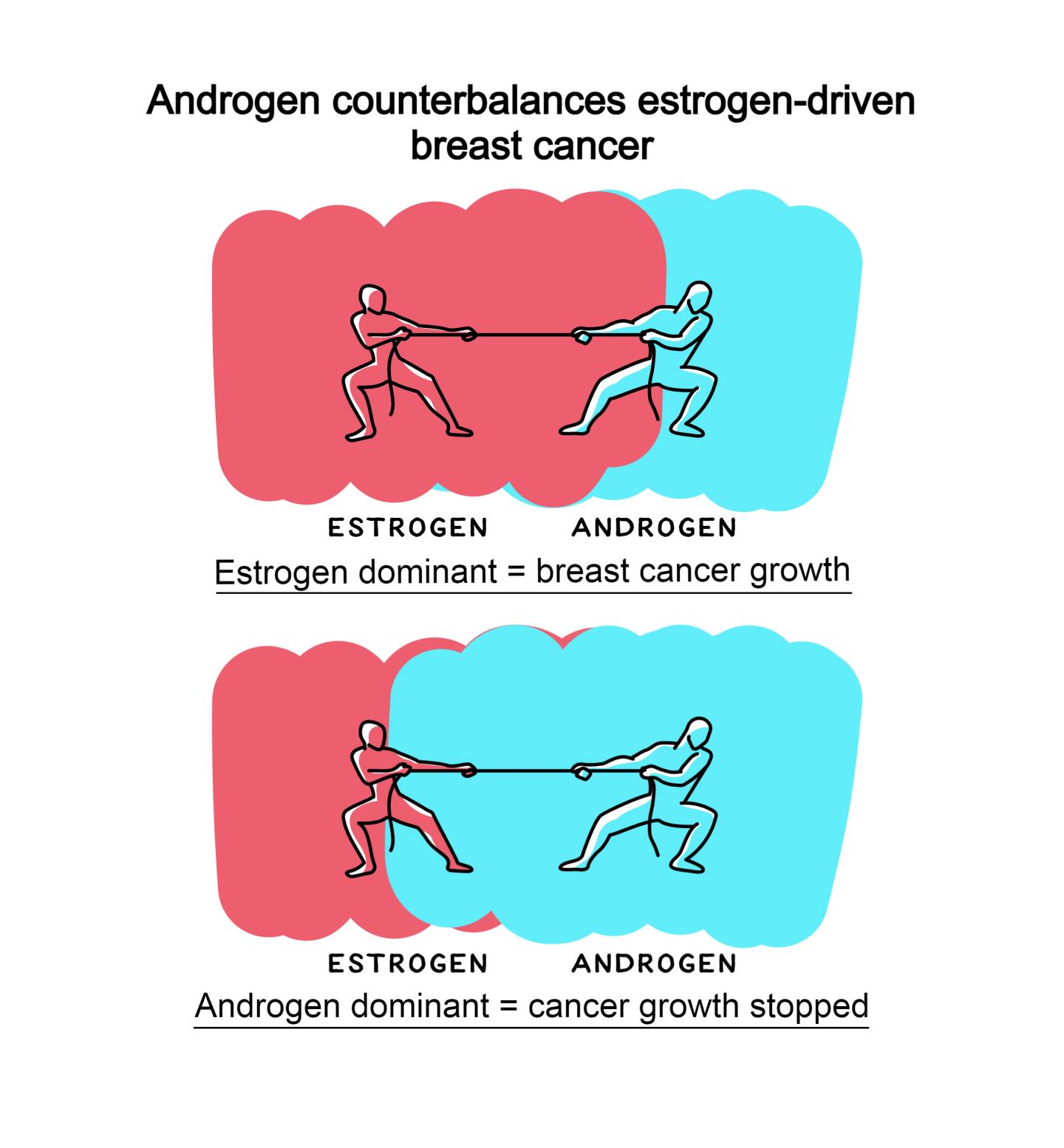
In normal breast development, estrogen stimulates and androgen inhibits growth at puberty and throughout adult life. Abnormal estrogen activity is responsible for the majority of breast cancers, but the role of androgen activity in this disease has been controversial.
Androgens were historically used to treat breast cancer, but knowledge of hormone receptors in breast tissue was rudimentary at the time and the treatment’s efficacy misunderstood. Androgen therapy was discontinued due to virilising side effects and the advent of anti-estrogenic endocrine therapies.
While endocrine therapy is standard-of-care for estrogen receptor positive breast cancer, resistance to these drugs are the major cause of breast cancer mortality.
Professor Wayne Tilley, Director of the Dame Roma Mitchell Cancer Research Laboratories, and Associate Professor Theresa Hickey, Head of the Breast Cancer Group, who led the study say the need for alternative treatment strategies has renewed interest in androgen therapy for breast cancer.
However, previous studies had produced conflicting evidence on how best to therapeutically target the androgen receptor for treatment of breast cancer, which caused widespread confusion and hampered clinical application.
Using cell-line and patient-derived models, a global team, including researchers at the University of Adelaide and the Garvan Institute, demonstrated that androgen receptor activation by natural androgen or a new androgenic drug had potent anti-tumour activity in all estrogen receptor positive breast cancers, even those resistant to current standard-of-care treatments. In contrast, androgen receptor inhibitors had no effect.
“This work has immediate implications for women with metastatic estrogen receptor positive breast cancer, including those resistant to current forms of endocrine therapy,” said Associate Professor Theresa Hickey.
Professor Tilley added: “We provide compelling new experimental evidence that androgen receptor stimulating drugs can be more effective than existing (e.g. Tamoxifen) or new (e.g. Palbociclib) standard-of-care treatments and, in the case of the latter, can be combined to enhance growth inhibition.
Moreover, currently available selective androgen receptor activating agents lack the undesirable side effects of natural androgens, and can confer benefits in women including promotion of bone, muscle and mental health.
Associate Professor Elgene Lim, a breast oncologist and Head of the Connie Johnson Breast Cancer Research Lab at the Garvan Institute, said: “The new insights from this study should clarify the widespread confusion over the role of the androgen receptor in estrogen receptor driven breast cancer. Given the efficacy of this treatment strategy at multiple stages of disease in our study, we hope to translate these findings into clinical trials as a new class of endocrine therapy for breast cancer.”
Dr Stephen Birrell, a breast cancer specialist and pioneer in androgens and women’s health who was part of the Adelaide based team, pointed out that this seminal finding has application beyond the treatment of breast cancer, including breast cancer prevention and treatment of other disorders also driven by estrogen.
Chloe Marshall, 33, has a breast cancer recurrence while pregnant with her second child. She said that endocrine therapy has terrible side effects and there was an urgent need for better options to prevent and treat breast cancer recurrence.
“I was diagnosed with a hormone positive breast cancer in July 2017 and subsequently found out I carried the BRACA gene,” she said.
“I underwent a double mastectomy and neo adjuvant chemotherapy followed by two years of hormone suppressive treatment. The hormone suppressive treatment that I experienced was one of the hardest parts of having cancer. The impact it has on your mind/life/body is incredibly challenging.
“Now, three years later, I find myself with a recurrent cancer while 25 weeks pregnant. The thought of having hormone suppressive treatment for a further five to ten years is overwhelming.
“I think this study will help patients like myself have hope that there is another answer to life after the cancer diagnosis.”
###
An international Phase 3 registration clinical trial (sponsored by VERU, Inc) evaluating Enobosarm, an androgen receptor activating agent, in patients with androgen receptor and estrogen receptor positive metastatic breast cancer who failed endocrine therapy and a CDK 4/6 inhibitor (e.g. palbociclib), will commence in the second quarter of 2021.
This work was funded by grants from the National Health and Medical Research Council of Australia, the National Breast Cancer Foundation, Cancer Australia, Movember, The Hospital Research Foundation and the US Department of Defense Breast Cancer Research Program.
Media Contact
Professor Wayne Tilley
[email protected]
Related Journal Article
http://dx.