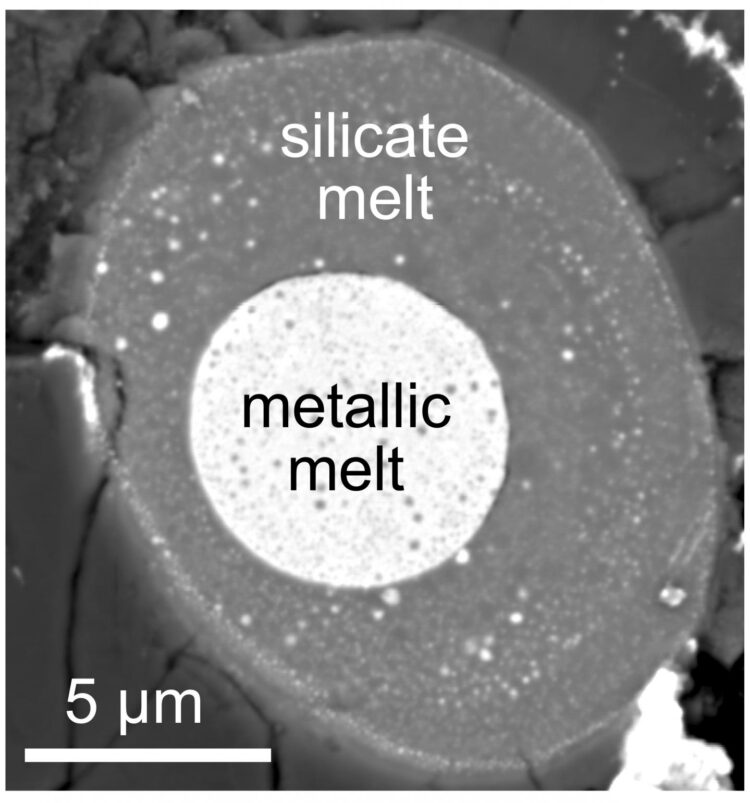
New work reveals how carbon behaved during Earth’s violent formative period
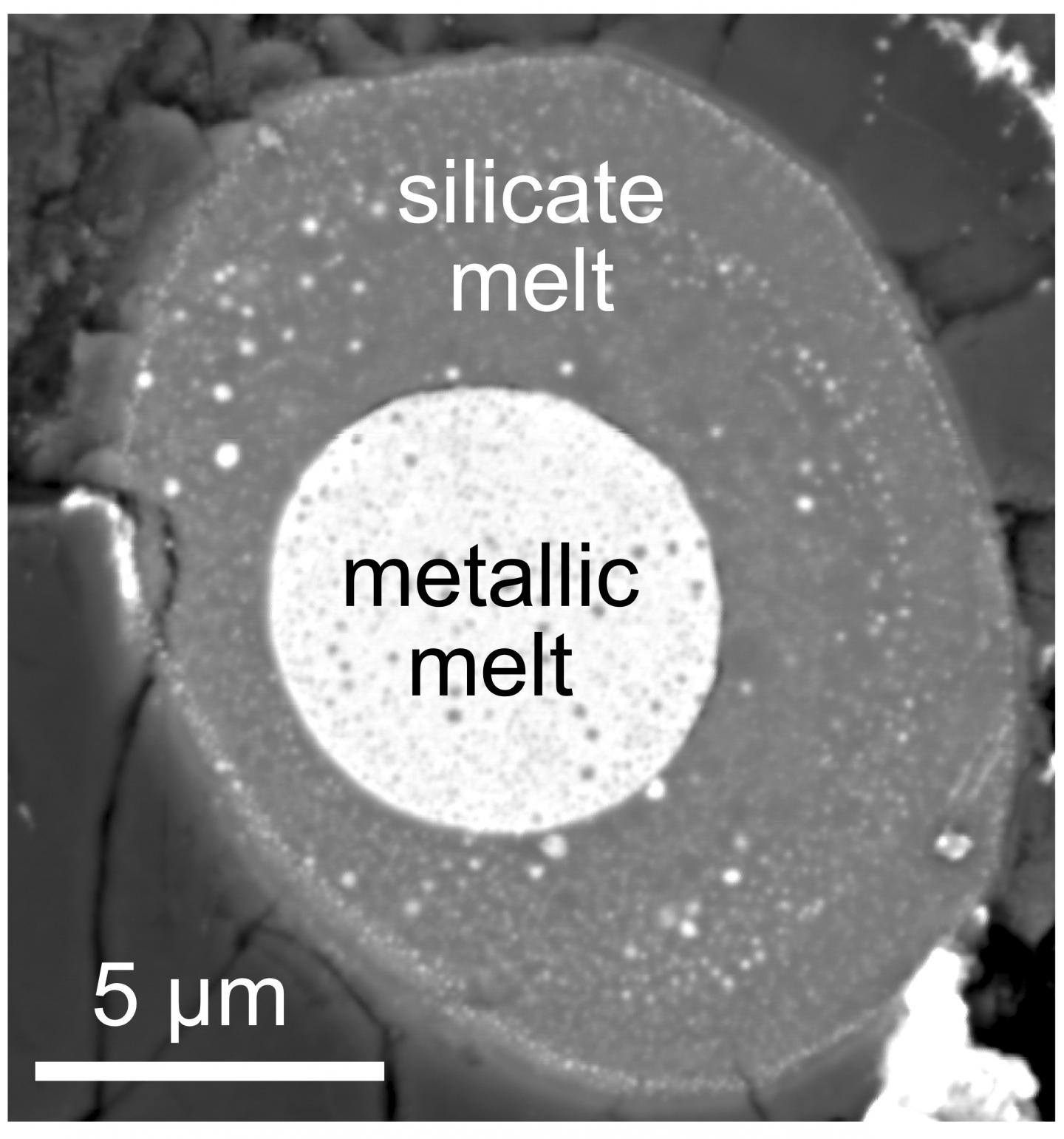
Credit: Rebecca Fischer, Elizabeth Cottrell and Marion Le Voyer, Kanani Lee, and the late Erik Hauri.
Washington, DC– Carbon is essential for life as we know it and plays a vital role in many of our planet’s geologic processes–not to mention the impact that carbon released by human activity has on the planet’s atmosphere and oceans. Despite this, the total amount of carbon on Earth remains a mystery, because much of it remains inaccessible in the planet’s depths.
New work published this week in Proceedings of the National Academy of Sciences reveals how carbon behaved during Earth’s violent formative period. The findings can help scientists understand how much carbon likely exists in the planet’s core and the contributions it could make to the chemical and dynamic activity occurring there–including to the convective motion powering the magnetic field that protects Earth from cosmic radiation.
Earth’s core is comprised mostly of iron and nickel, but its density indicates the presence of other lighter elements, such as carbon, silicon, oxygen, sulfur, or hydrogen. It’s been long suspected that there’s a tremendous reservoir of carbon hiding down there. But to attempt to quantify it, the research team used laboratory mimicry to understand how it got into the core in the first place.
The group was comprised of Harvard University’s Rebecca Fischer, the Smithsonian Institution’s Elizabeth Cottrell and Marion Le Voyer, both former Carnegie postdoctoral fellows, Yale University’s Kanani Lee, and Carnegie’s late Erik Hauri, the memory of whom the authors acknowledge.
“To understand present day Earth’s carbon content, we went back to our planet’s babyhood, when it accreted from material surrounding the young Sun and eventually separated into chemically distinct layers–core, mantle, and crust,” said Fischer. “We set out to determine how much carbon entered the core during these processes.”
This was accomplished by lab experiments that compared carbon’s compatibility with the silicates that comprise the mantle to its compatibility with the iron that comprises the core while under the extreme pressures and temperatures found deep inside the Earth during its formative period.
“We found that more carbon would have stayed in the mantle than we previously suspected,” explained Cottrell. “This means the core must contain significant amounts of other lighter elements, such as silicon or oxygen, both of which become more attracted to iron at high temperatures.”
Despite this surprising discovery, the majority of Earth’s total carbon inventory does likely exist down in the core. But it still makes up only a negligible component of the core’s overall composition.
“Overall, this important work improves our understanding of how Earth’s carbon was accumulated during the planetary formation process and sequestered into the mantle and core as they chemically differentiated,” concluded Richard Carlson, Director of Carnegie’s Earth and Planets Laboratory, where Hauri worked. “I only wish Erik was still with us to see the results published this week.”
###
This work was supported by a National Science Foundation postdoctoral fellowship.
It was performed, in part, under the auspices of the U.S, Department of Energy by Lawrence Livermore National Laboratory.
The Carnegie Institution for Science (carnegiescience.edu) is a private, nonprofit organization headquartered in Washington, D.C., with six research departments throughout the U.S. Since its founding in 1902, the Carnegie Institution has been a pioneering force in basic scientific research. Carnegie scientists are leaders in plant biology, developmental biology, astronomy, materials science, global ecology, and Earth and planetary science.
Media Contact
Liz Cottrell
[email protected]
Related Journal Article
http://dx.