Early response noted days after treatment
Credit: Images created by E. O’Neill et al., University of Oxford, United Kingdom.
A new proof-of-concept study published in the May issue of The Journal of Nuclear Medicine has demonstrated that molecular imaging can be used for identifying early response to 177Lu-DOTATATE treatment in neuroendocrine tumor patients.
Utilizing single photon emission computed tomography (SPECT) imaging with 111In-antiγH2AX-TAT, researchers were able to visualize a DNA damage response marker just days after 177Lu-DOTATATE treatment. Monitoring the DNA damage response in the early days after the radionuclide injection could allow physicians to determine the therapeutic outcome and adapt the therapy regimen accordingly.
The radiobiologic aspects of 177Lu-DOTATATE, as well as other molecular radiotherapies, are underexplored. Radionuclide therapy is largely delivered to neuroendocrine tumor patients on a fixed dose protocol, regardless of body weight or tumor uptake. To justify any increase or decrease in the prescribed radionuclide dose a sustainable metric is needed; however, no metric currently exists.
“One strategy to develop this metric is to determine if sufficient damage has been afflicted to the tumor, which would allow treating physicians to tailor subsequent doses to ensure therapeutic success,” said Bart Cornelissen, PhD, associate professor in the department of oncology at the MRC Oxford Institute for Radiation Oncology at the University of Oxford in Oxford, United Kingdom. “In our study, we sought to image the molecular biological effects of 177Lu-DOTATATE radionuclide therapy by visualizing the DNA double-strand break damage response marker γH2AX.”
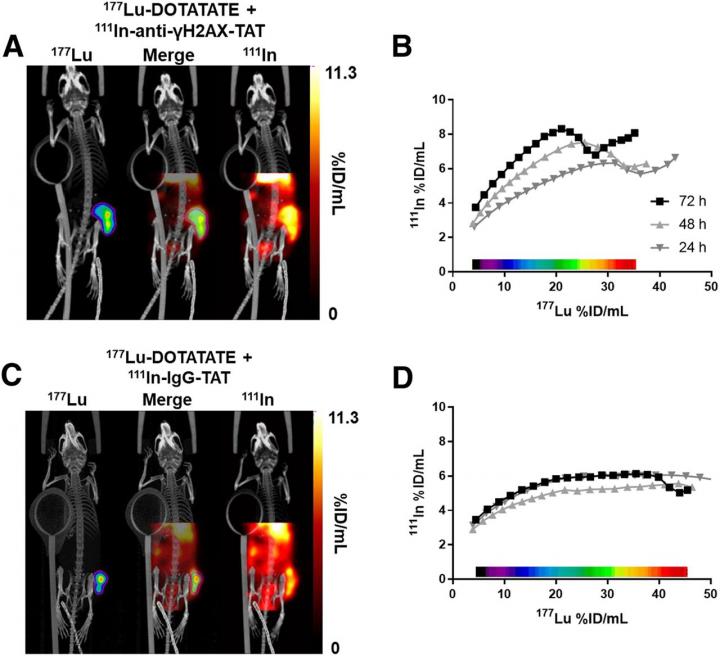
In the study, researchers first exposed six cell lines to external beam therapy or 177Lu-DOTATATE and measured the number of γH2AX foci and the clonogenic survival (which indicate the extent of DNA double-strand break damage). Mice bearing the same cell line were then treated with 177Lu-DOTATATE or sham-treated, and static SPECT images were acquired at one, 24, 48 and 72 hours after administration. Immediately after the first SPECT imaging session, the mice were administered 111In-anti-γH2AX-TAT or 111In-IgG-TAT.
In vitro cell lines exposed to 177Lu-DOTATATE were found to have increased γH2AX foci and decreased clonogenic survival, and reacted very differently than to an equitoxic dose of external beam irradiation. The γH2AX foci induced by 177Lu-DOTATATE in the preclinical models were successfully imaged by SPECT in vivo using 111In-anti-γH2AX-TAT. An accumulation of γH2AX signal was observed over the days after administration of 177Lu-DOTATATE, indicating an increase in DNA damage. Furthermore, γH2AX expression revealed intratumoral and interlesion heterogeneity with the absorbed 177Lu dose, suggesting that different parts of the tumor may react differentially to treatment with 177Lu-DOTATATE.
“The application of this imaging technique could provide a very early indicator of tumor damage without having to wait for changes in tumor volume, which currently may take months to find out,” noted Edward O’Neill, postdoctoral researcher in the department of oncology at MRC Oxford Institute for Radiation Oncology at the University of Oxford in Oxford, United Kingdom. “When using therapeutic response assessment with molecular imaging, making rapid decisions becomes possible, including dose reduction to avoid side effects, assessment of combination therapies, or, in the absence of any measurable response, initiation of palliative options designed toward improving quality of life.”
###
This study was made available online in November 2019 ahead of final publication in print in May 2020.
The authors of “Imaging DNA Damage Repair In Vivo After 177Lu-DOTATATE Therapy” include Edward O’Neill, Veerle Kersemans, P. Danny Allen, Julia Baguña Torres, Michael Mosely, Sean Smart, Boon Quan Lee, Nadia Falzone, Katherine A. Vallis and Bart Cornelissen, CRUK/MRC Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom; Samantha Y.A. Terry, Department of Imaging Chemistry and Biology, King’s College London, London, United Kingdom; Mark W. Konijnenberg and Marion de Jong, Department of Radiology and Nuclear Medicine, Erasmus MC, Rotterdam, The Netherlands; and Julie Nonnekens, Department of Radiology and Nuclear Medicine, Erasmus MC, Rotterdam, The Netherlands, Department of Molecular Genetics, Erasmus MC, Rotterdam, The Netherlands, and Oncode Institute, Erasmus MC, Rotterdam, The Netherlands.
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected]. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.
About the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed close to 10 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging–precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit http://www.
Media Contact
Rebecca Maxey
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.