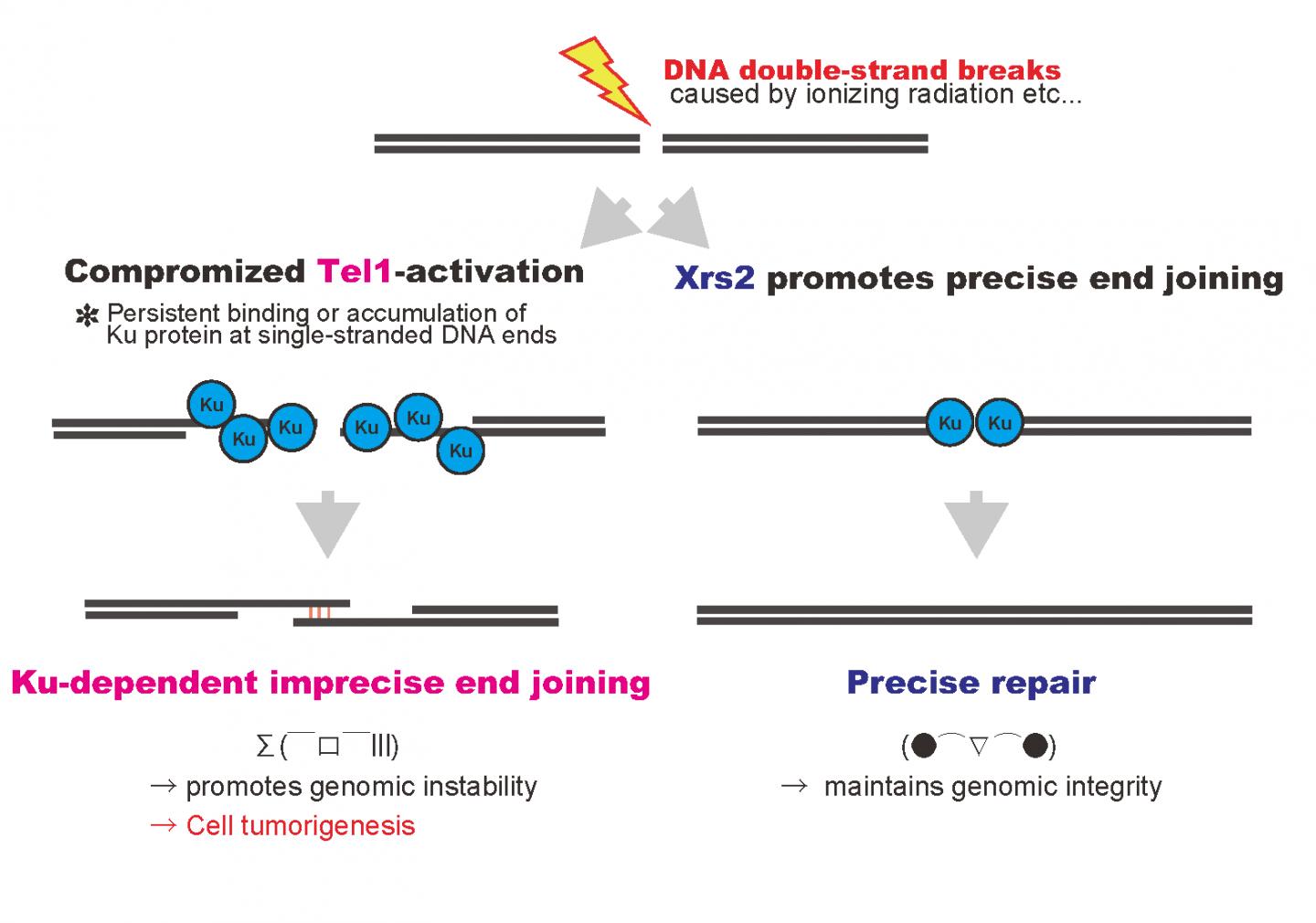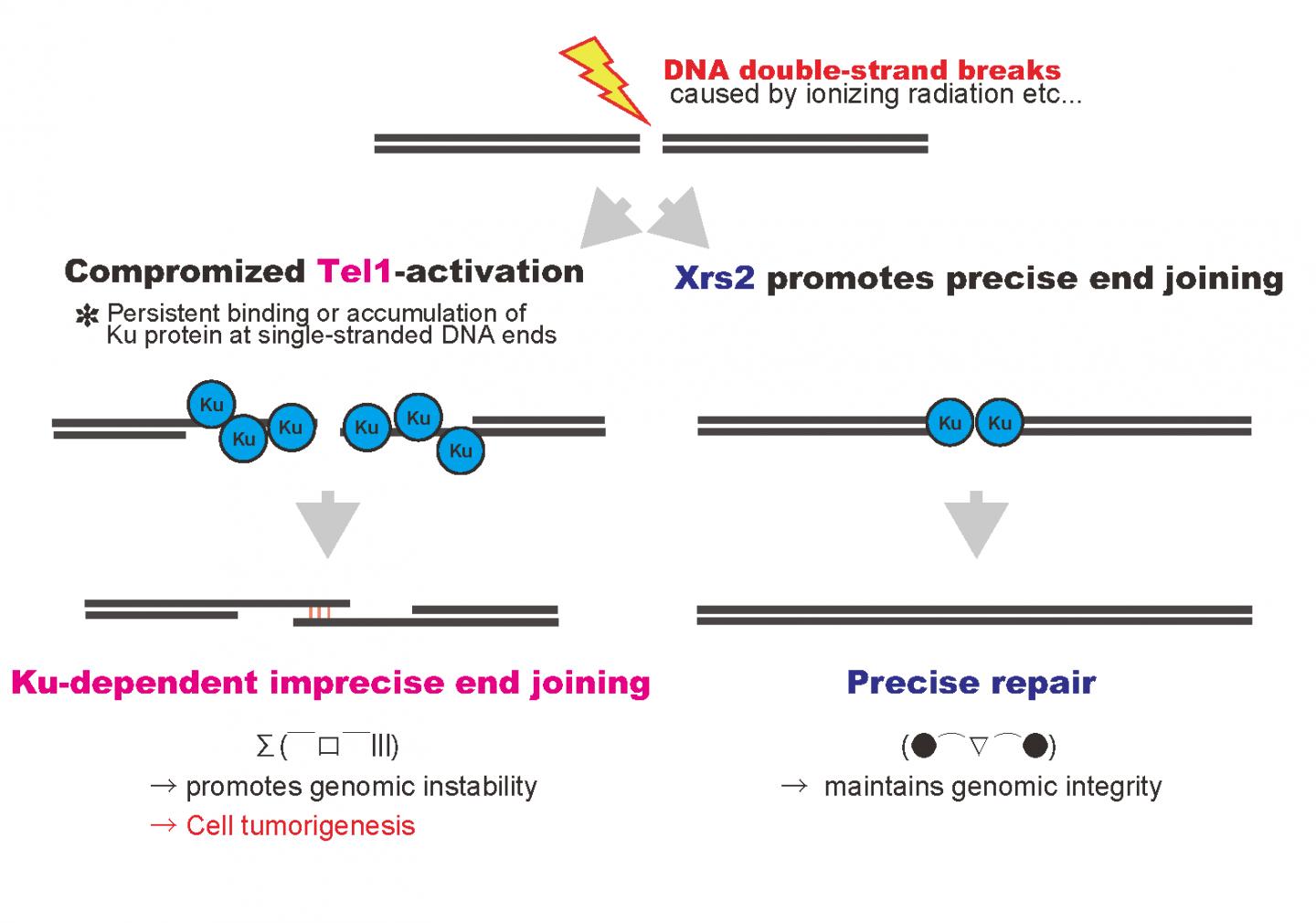
A group of researchers at Osaka University found that if DNA damage response (DDR) does not work when DNA is damaged by radiation, proteins which should be removed remain instead, and a loss of genetic information can be incited, which, when repaired incorrectly, will lead to the tumor formation.
It is thought that one of the reasons why cells become cancerous is that the source of genetic information is physically destroyed. Our body has a system to repair DNA damage (DNA repair mechanism), so why a normal cell turns into a cancer cell and why radiation exposure causes cancer have not been clarified.
Radiation damages genomic DNA, the essential blueprint for life; therefore, living organisms have several mechanisms for maintaining the stability of their own genomes. Although they have big evolutionary differences, both humans and budding yeast contain proteins that perform the same function. Miki Shinohara, associate professor and her group at the Institute for Protein Research, Osaka University examined the DNA repair function of yeast Xrs2, an orthlog of human Nbs1, and yeast Tel1, an orthlog of human ATM.
Mutations in the Nbs1 gene are responsible for a human hereditary disorder which develops a high risk of cancer. This group found that DNA damage was repaired when human hereditary disorder type mutations (xrs2 mutations) were introduced in yeast XRS2 genes, but it was repaired with more errors than a DNA sequence with no mutations. This group determined that the cause was that the function of the Tel1 protein, which is important for DNA damage response, was not fully implemented in xrs2 mutations.
In the process of DNA repair through homologous recombination, it is necessary to make double-stranded DNA near DNA lesions into single-stranded DNA. This group clarified that Ku remained on DNA damage in tel1 mutants and xrs2 mutants. Ku is not required, so it should be removed when DNA damage is repaired.
Ku is a protein to join DNA ends broken by non-homologous end joining (NHEJ). It is thought that Ku, a repair tool, joins DNA ends where Ku normally should not work, and as a result, repair is completed with incorrect DNA information.
Tumor formation occurs when genomic DNA is broken or errors have become continuous. In human cells as well, if Nbs1 and ATM function in the same way to ensure repair of DNA damage, tumor formation may be prevented.
This group’s achievement shows the possibility to clarify the mechanism of human tumor formation, especially the molecular mechanism responsible for in the initial stage of cell cancerization due to DNA damaged by radiation in the initial stage, by using the model of budding yeast, a primitive eukaryote. Furthermore, it may be possible to clarify the molecular mechanism of cancerization by radiation exposure by verifying it using human cells.
###
This research was featured in the electronic version of PLOS Genetics on Saturday, March 19, 2016.
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
@osaka_univ_e
http://www.osaka-u.ac.jp/en
The post May repairs full of mistakes develop into cancer? appeared first on Scienmag.