Researchers led by a team at the University of Tsukuba find that hair-like structures on the surface of aquatic bacterium Leptothrix cholodnii aren’t simply for aesthetics — they are essential for niche establishment and can capture precious metals
Credit: University of Tsukuba
Tsukuba, Japan – When you’re only one of trillions, it can be hard to get ahead. That’s the problem faced by aquatic bacterium Leptothrix cholodnii, which is often found in the slime-like microbial mats common to mineral-rich bodies of water. Therefore, to establish itself in these communities, L. cholodnii forms long, rigid filaments that become an integral part of the structure of the microbial mat.
In a study published last week in ACS Nano, a team led by researchers from the University of Tsukuba used microfluidic chambers to enable visualization of L. cholodnii and study the contribution of nanofibrils to filament formation. A deeper understanding of this process could help researchers make significant inroads in the use of sheath-forming bacteria for processes such as the development of novel amorphous iron oxides for lithium-ion battery anodes and the industrial harvesting of pigments and heavy metals.
L. cholodnii filaments are composed of chains of cells initially surrounded by a soft sheath made of thousands of tiny intertwined hair-like structures called nanofibrils. During filament formation, the bacteria release proteins that oxidize iron and manganese in the water, producing metal oxides that accumulate in the nanofibrils, causing them to harden into a microtube. The nanofibrils can also incorporate precious metals such as gold, silver, titanium, and zirconium. However, the exact role of nanofibrils in filament formation is not known.
“Because microbial mats are often found on stream beds, we used microfluidic chambers to replicate the running water found in these locations,” explains lead author of the study Tatsuki Kunoh. “We allowed individual cells to pass into the chambers and then used time-lapse and intermittent fluorescent straining of nanofibrils and atmospheric scanning electron microscopy to examine the behaviour of individual cells and the developing multicellular filaments.”
The researchers showed that nanofibrils are essential for bacterial cell attachment to solid surfaces, which is required for filament formation. Confirming this observation, variant “sheathless” L. cholodnii cells, which did not produce nanofibrils, roamed around the chambers throughout the experiment, unable to attach or form a filament.
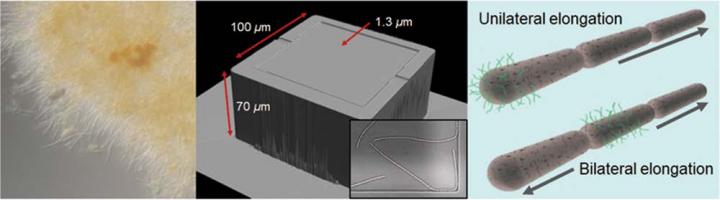
“By fluorescently staining the nanofibrils, we could monitor their distribution on the bacterial cell surface,” says Dr Kunoh. “Interestingly, the positioning of the nanofibrils seemed to dictate the direction of filament elongation–during unilateral elongation, nanofibrils were clustered around the non-dividing end of the cell, while in bilateral elongation, nanofibrils were only present around the central portion of the cell.”
The researchers also observed that nanofibrils were densely woven around the mature sections of the growing filaments but formed a more open web-like structure around the newly divided cells.
These new insights into the role of nanofibrils in the development of filaments could enable researchers to tailor L. cholodnii for use in industrial applications such as bioremediation, the extraction of heavy and precious metals, and microwire fabrication.
###
Media Contact
Naoko Yamashina
[email protected]
81-298-532-066
Related Journal Article
http://dx.




