Phase 1 trials conducted at NIH Clinical Center
Credit: NIAID
WHAT:
Two U.S. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were later exposed to disease-causing malaria parasites. The vaccine combines live parasites with either of two widely used antimalarial drugs–an approach termed chemoprophylaxis vaccination. A Phase 2 clinical trial of the vaccine is now underway in Mali, a malaria-endemic country. If the approach proves successful there, chemoprophylaxis vaccination, or CVac, potentially could help reverse the stalled decline of global malaria. Currently, there is no vaccine in widespread use for the mosquito-transmitted disease.
The trials were conducted at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. They were led by Patrick E. Duffy, M.D., of the NIH National Institute of Allergy and Infectious Diseases (NIAID), and Stephen L. Hoffman, M.D., CEO of Sanaria Inc., Rockville, Maryland.
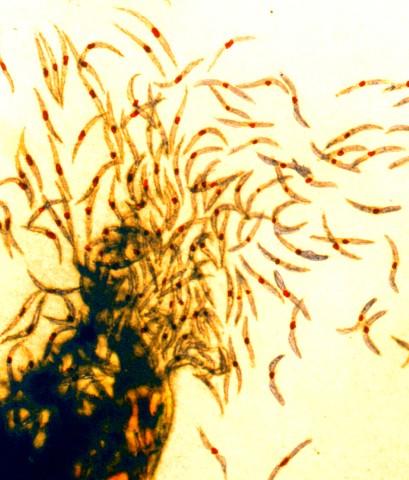
The Sanaria vaccine, called PfSPZ, is composed of sporozoites, the form of the malaria parasite transmitted to people by mosquito bites. Sporozoites travel through blood to the liver to initiate infection. In the CVac trials, healthy adult volunteers received PfSPZ along with either pyrimethamine, a drug that kills liver-stage parasites, or chloroquine, which kills blood-stage parasites. Three months later, under carefully controlled conditions, the volunteers were exposed to either an African malaria parasite strain that was the same as that in the vaccine (homologous challenge) or a variant South American parasite (heterologous challenge) that was more genetically distant from the vaccine strain than hundreds of African parasites. Exposure in both cases was via inoculation into venous blood, which infects all unvaccinated individuals.
At the lowest PfSPZ dosage, the CVac approach conferred modest protection: only two of nine volunteers (22.2%) who received the pyrimethamine combination were protected from homologous challenge. In contrast, seven out of eight volunteers (87.5%) who received the highest PfSPZ dosage combined with pyrimethamine were protected from homologous challenge, and seven out of nine volunteers (77.8%) were protected from heterologous challenge. In the case of the chloroquine combination, all six volunteers (100%) who received the higher PfSPZ dosage were completely protected from heterologous challenge. The high levels of cross-strain protection lasted at least three months (the time elapsed between vaccination and challenge) for both higher-dose regimens. One hundred percent protection for three months against heterologous variant parasites is unprecedented for any malaria vaccine in development, the authors note. These data suggest that CVac could be a promising approach for vaccination of travelers to and people living in malaria-endemic areas.
###
Additional information about the trials is available at ClinicalTrials.gov using the identifiers NCT02511054 and NCT03083847.
ARTICLE: A Mwakingwe-Omari et al. Two chemoattenuated PfSPZ malaria
vaccines induce sterile hepatic immunity
. Nature DOI: 10.1038/s41586-021-03684-z (2021).
WHO:
Dr. Duffy, chief of the Laboratory of Malaria Immunology and Vaccinology, NIAID, is available to discuss this research.
CONTACT:
To schedule interviews, please contact Anne A. Oplinger, (301) 402-1663, [email protected].
This work was supported by the NIAID Intramural Research Program and by the NIAID grants U01AI109700-01 and 2R44AI058375-11 to Sanaria for vaccine manufacture and trial execution.
NIAID conducts and supports research–at NIH, throughout the United States, and worldwide–to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.
Media Contact
Anne A. Oplinger
[email protected]
Related Journal Article
http://dx.




