Scientists have found catalysts that improve an important industrial reaction and make it more eco-friendly
Credit: Kazuaki Ishihara
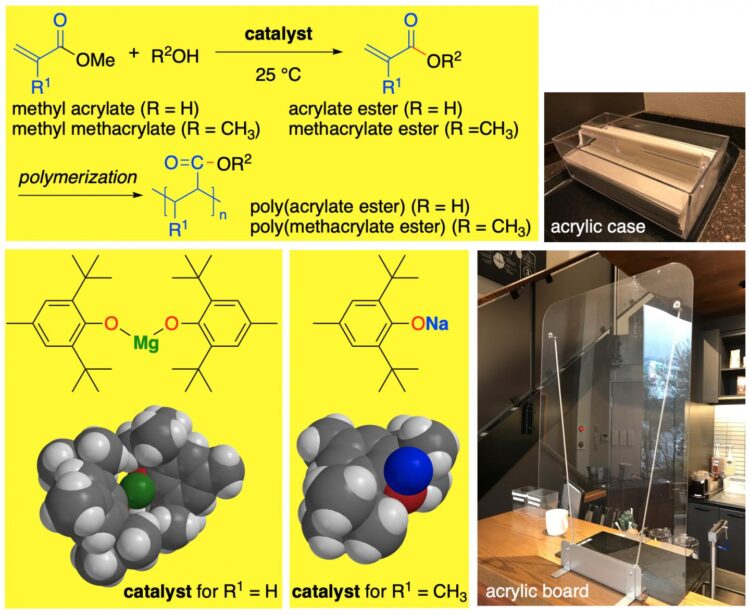
Nagoya University scientists have developed a chemical reaction that produces high yields of a compound used in a wide variety of industries, without needing high temperatures or toxic catalysts. The approach was described in the journal ACS Catalysis and offers a practical and sustainable solution for industrial (meth)acrylate (= acrylate or methacrylate) ester synthesis.
(Meth)acrylate esters are used in industrial coatings and masonry, and to make plastics, dyes and adhesives. But the chemical process for making them from methyl (meth)acrylates involves high temperatures, long reaction times and toxic compounds. It can also result in unwanted side reactions.
Scientists, including Nagoya University professor Kazuaki Ishihara and colleagues, have been working on improving this process to make it more eco-friendly. Specifically, they worked on improving the catalyst involved in the chemical reaction that turns methyl (meth)acrylates into (meth)acrylate esters, called transesterification.
“Millions of tons of (meth)acrylate esters are produced annually and are among the most important manufactured chemicals around,” says Ishihara. “Their transesterification, using alcohol and a catalyst, fine-tunes their properties, producing a wide range of (meth)acrylate esters.”
Ishihara and his colleagues found that sterically bulky sodium and magnesium aryloxides worked very well as non-toxic alternatives. They catalysed the transesterification of methyl (meth)acrylates at the relatively mild temperature of 25°C, producing high yields of a broad range of (meth)acrylate esters depending on the type of alcohol used in the reaction.
The team also conducted computational calculations to uncover the details of what happened during the chemical reaction, showing that it had high chemoselectivity; in other words, the reaction happened the way the scientists wanted it to without having undesirable side reactions.
“Our transesterification process is a practical and sustainable candidate for industrial (meth)acrylate ester synthesis, providing excellent chemoselectivity, high yields, mild reaction conditions and a lack of any toxic metal salts,” says Ishihara.
The team next aims to collaborate with colleagues in industry to use their approach in (meth)acrylate ester production. They also aim to continue searching for efficient catalysts for the transesterification of methyl (meth)acrylates and to develop recyclable catalysts.
###
The study, “Chemoselective Transesterification of Methyl (Meth)acrylates Catalyzed by Sodium(I) or Magnesium(II) Aryloxides,” was published online in ACS Catalysis on December 16, 2020 at DOI: 10.1021/acscatal.0c04217.
About Nagoya University, Japan
Nagoya University has a history of about 150 years, with its roots in a temporary medical school and hospital established in 1871, and was formally instituted as the last Imperial University of Japan in 1939. Although modest in size compared to the largest universities in Japan, Nagoya University has been pursuing excellence since its founding. Six of the 18 Japanese Nobel Prize-winners since 2000 did all or part of their Nobel Prize-winning work at Nagoya University: four in Physics – Toshihide Maskawa and Makoto Kobayashi in 2008, and Isamu Akasaki and Hiroshi Amano in 2014; and two in Chemistry – Ryoji Noyori in 2001 and Osamu Shimomura in 2008. In mathematics, Shigefumi Mori did his Fields Medal-winning work at the University. A number of other important discoveries have also been made at the University, including the Okazaki DNA Fragments by Reiji and Tsuneko Okazaki in the 1960s; and depletion forces by Sho Asakura and Fumio Oosawa in 1954.
Website: http://en.
Media Contact
Kazuaki Ishihara
[email protected]
Original Source
http://en.
Related Journal Article
http://dx.